
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2024)Volume 15, Issue 6
Triple Negative Breast Cancer (TNBC) is an aggressive subtype of breast cancer characterized by the absence of estrogen receptors, progesterone receptors and Human Epidermal growth factor Receptor 2 (HER2) amplification. This study aimed to identify prognostic and predictive biomarkers associated with disease progression in TNBC. We compared glycolysis indicators, inflammatory markers, neurotransmitters, Matrix Metalloproteinases (MMPs) and adaptive biomarkers across three groups such as patients with advanced TNBC, non-advanced TNBC (T1N0M0G1) and healthy controls.
Our results showed significant metabolic reprogramming in TNBC, with elevated lactate and pyruvate levels indicating a reliance on glycolysis. Inflammatory markers such as Interleukin-6 (IL-6) and Tumor Necrosis Factor (TNF) alpha were markedly increased in advanced TNBC, reflecting a chronic inflammatory state that supports tumor progression. Neurotransmitter levels, particularly dopamine and serotonin, were critically low in advanced TNBC patients, suggesting severe neuroendocrine disruption. Elevated levels of MMP-2 and MMP-9 were associated with enhanced extracellular matrix degradation and a high metastatic potential.
The study also highlighted the role of hepcidin, a regulator of iron metabolism, in TNBC. Elevated hepcidin levels were linked to anaemia of chronic disease and the potential use of hepcidin inhibitors in combination with chemotherapy was explored as a therapeutic strategy. Survival analysis indicated that patients with irreversible increases in these biomarkers had significantly shorter survival times, underscoring their prognostic significance.
This study provides a comprehensive analysis of key biomarkers in TNBC, offering insights into their roles in disease progression and potential therapeutic targets. Future research should focus on validating these biomarkers and exploring novel therapeutic approaches to improve outcomes for TNBC patients.
Prognostic biomarkers; Glycolysis; Hepcidin inhibitors; Inflammatory markers; Melatonin; Dopamine
Triple Negative Breast Cancer (TNBC) is a particularly aggressive subtype of breast cancer that lacks expression of Estrogen Receptors (ER), Progesterone Receptors (PR) and Human Epidermal growth factor Receptor 2 (HER2). This absence of targeted receptors makes TNBC more challenging to treat compared to other breast cancer subtypes, as there is no specific hormonal or targeted therapies available. TNBC accounts for approximately 10%-15% of all breast cancers and is often associated with a poorer prognosis due to its aggressive nature, higher recurrence rates and a tendency to metastasize early [1].
One of the distinctive feature of TNBC is the metabolic reprogramming of cancer cells, characterized by increased glycolysis even in the presence of oxygen, a phenomenon known as the Warburg effect. This metabolic shift supports rapid cell proliferation and survival within the hypoxic tumor microenvironment but also leads to significant alterations in systemic biomarkers. Alongside metabolic changes, TNBC is closely associated with chronic inflammation, which contributes to tumor progression and resistance to treatment. Inflammatory cytokines, such as Interleukin-6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-alpha), play pivotal roles in creating a tumor-promoting environment that supports cancer growth and metastasis.
Additionally, TNBC affects neurotransmitter levels, particularly dopamine and serotonin, as well as melatonin, a hormone with oncostatic properties. These changes are linked to the neuroendocrine disruption observed in TNBC patients, which can influence tumor behavior and patient symptoms. Matrix Metalloproteinases (MMPs), which are involved in the degradation of the Extracellular Matrix (ECM), further contribute to the invasive and metastatic potential of TNBC cells by facilitating tumor invasion and metastasis [2].
This study aims to identify a set of prognostic and predictive biomarkers that can indicate the irreversibility of the disease process in patients with advanced TNBC. We compared glycolysis indicators, inflammatory markers, neurotransmitters, MMPs and adaptive biomarkers between patients with advanced TNBC (TxNxM1G3), non-advanced TNBC (T1N0M0G1) and a control group of healthy women. The ultimate goal is to better understand the role of these biomarkers in TNBC progression and their potential use in guiding treatment strategies, including the possible combination of hepcidin inhibitors with chemotherapy.
Hepcidin, a key regulator of iron metabolism, has emerged as an important biomarker in cancer research. Elevated hepcidin levels have been observed in various cancers, including TNBC, where it contributes to anemia of chronic disease and may support tumor growth by manipulating iron availability. The potential use of hepcidin inhibitors, which counteract the effects of elevated hepcidin, could offer a novel therapeutic approach, especially when combined with chemotherapy. This combination could alleviate anemia, enhance chemotherapy efficacy and potentially modulate the tumor microenvironment to inhibit cancer progression.
In this comprehensive study, we will explore the roles of glycolysis indicators, inflammatory markers, neurotransmitters, MMPs and adaptive biomarkers, particularly focusing on hepcidin and its antagonists, in TNBC. We will assess the impact of these biomarkers on patient outcomes and survival rates, with a specific focus on patients exhibiting critical increases in these markers, which may indicate irreversible disease progression. The results will be discussed in the context of potential treatment strategies, including the feasibility and benefits of combining hepcidin inhibitors with chemotherapy to improve patient outcomes in TNBC. Relevant data and findings will be presented in tables and graphs throughout the article, with specific references to these visual aids to support the discussion [3,4].
Study design
This comparative study was designed to evaluate and compare various biomarkers among three distinct groups such as patients with advanced TNBC (TxNxM1G3), patients with non-advanced TNBC (T1N0M0G1) and a control group of healthy women. The primary objective was to identify prognostic and predictive biomarkers that could indicate irreversible disease progression in advanced TNBC.
Participants: The study included 79 participants divided into three groups.
(a) Advanced TNBC group: 27 patients with advanced TNBC (TxNxM1G3).
(b) Non-advanced TNBC group: 27 patients with non-advanced TNBC (T1N0M0G1).
(c) Control group: 25 healthy women.
The age range of the participants was 26-73 years. All participants provided informed consent and the study was conducted in accordance with ethical guidelines [5-7].
Biomarker measurement
Blood and 24-hour urine samples were collected from all participants to measure a comprehensive set of biomarkers, focusing on glycolysis indicators, inflammatory markers, neurotransmitters, Matrix Metalloproteinases (MMPs) and adaptive biomarkers. The following biomarkers were measured using the described laboratory methods.
Glycolysis indicators: These include blood glucose, lactate, pyruvate, insulin, C-peptide, blood pH, ketone bodies, serum iron and hepcidin.
(a) Blood glucose: Measured using enzymatic assays.
(b) Lactate: Measured using enzymatic assays.
(c) Pyruvate: Assessed using a colorimetric assay.
(d) Insulin: Quantified using Enzyme-Linked Immunosorbent Assay (ELISA).
(e) C-peptide: Quantified using ELISA.
(f) Blood pH: Determined using an arterial blood gas analyzer.
(g) Ketone bodies: Measured via enzymatic methods.
(h) Serum iron: Measured using atomic absorption spectrophotometry.
(i) Hepcidin: Measured using ELISA.
Inflammatory markers: These include interleukin-6 and tumor necrosis factor-alpha.
(a) Interleukin-6 (IL-6): Quantified using ELISA.
(b) Tumor Necrosis Factor-Alpha (TNF-alpha): Quantified using ELISA.
Neurotransmitters: These include dopamine and serotonin.
(a) Dopamine: Levels in blood and 24-hour urine were measured using High Performance Liquid Chromatography (HPLC).
(b) Serotonin: Levels in blood and 24-hour urine were measured using HPLC.
Matrix Metalloproteinases (MMPs): These include MMP-1, MMP-2, MMP-3, MMP-7, MMP-9 of which levels were measured using multiplex ELISA.
Adaptive biomarkers: These include melatonin and melatonin sulfate.
(a) Melatonin: Measured in blood using radioimmunoassay.
(b) Melatonin sulfate: Measured in 24-hour urine using radioimmunoassay.
All medical reagents and standards were supplied and standardized by Foconsci Chemical Industry Co., Ltd/ShengPeng group.
Statistical analysis
Data analysis was performed using Statistical Package for Social Sciences (SPSS) software (version 25.0). The following statistical methods were employed.
Descriptive statistics: Used to summarize the data for all measured biomarkers.
Comparative analysis: One-way Analysis of Variance (ANOVA) was conducted to compare the biomarker levels across the three groups (advanced TNBC, non-advanced TNBC and healthy control). Post hoc Tukey tests were used to determine specific group differences.
Correlation analysis: Pearson’s correlation coefficient was used to assess the relationships between different biomarkers.
Survival analysis: For patients with TNBC, survival rates were analyzed using simulated data, comparing the group with irreversible changes (12 patients with critical increases in biomarkers) to the rest of the TNBC patients. Box plots were used to visualize the survival time distributions between the two groups.
A p-value of <0.05 was considered statistically significant for all analyses. The study’s findings are presented in a series of tables and graphs that highlight key differences in biomarker levels between the groups, as well as the survival rates associated with these biomarkers.
Hepcidin and its antagonists in the study
The role of hepcidin as a key regulator of iron metabolism was particularly emphasized in this study. Hepcidin levels were evaluated across the groups and the potential impact of hepcidin antagonists on TNBC treatment was discussed. The hypothesis that combining hepcidin inhibitors with chemotherapy could enhance treatment efficacy, reduce anemia and improve overall outcomes for TNBC patients was explored. This concept was examined by analyzing the relationship between hepcidin levels, iron metabolism and treatment responses, with particular attention to patients exhibiting irreversible changes in biomarker levels [8-10].
The next section will present the results of these analyses, along with detailed discussions on the implications of these findings for TNBC treatment strategies.
Patient demographics
The study included a total of 79 participants, divided into three groups: 27 patients with advanced TNBC (TxNxM1G3), 27 patients with non-advanced TNBC (T1N0M0G1) and 25 healthy controls. The mean age was 51.3 ± 10.4 years for the advanced TNBC group, 50.1 ± 9.8 years for the non-advanced TNBC group and 49.6 ± 10.1 years for the control group. There were no significant differences in age between the groups (p>0.05), ensuring that age was not a confounding factor in the analysis.
Glycolysis indicators
Blood glucose: The mean blood glucose levels were significantly lower in the advanced TNBC group (67 ± 8 mg/dL) compared to the non-advanced TNBC group (85 ± 10 mg/dL) and the control group (90 ± 8 mg/dL, p<0.01). This suggests a higher glucose consumption rate by the tumors in advanced TNBC, consistent with the Warburg effect (Table 1).
| Biomarker | Normal range | Advanced Triple Negative Breast Cancer (TNBC) (TxNxM1G3) |
Non-advanced TNBC (T1N0M0G1) | Healthy control |
|---|---|---|---|---|
| Blood glucose (mg/dL) | 70-99 | 67 ± 8 | 85 ± 10 | 90 ± 8 |
| Lactate (mmol/L) | 0.5-2.2 | 5.2 ± 0.7 | 3.8 ± 0.5 | 1.5 ± 0.4 |
| Pyruvate (mmol/L) | 0.08-0.1 | 0.9 ± 0.2 | 0.7 ± 0.1 | 0.4 ± 0.1 |
| Insulin (µU/mL) | 2-25 | Variable | Normal | Normal |
| C-peptide (ng/mL) | 0.5-2 | Variable | Normal | Normal |
| Blood pH | 7.35-7.45 | 7.28 ± 0.05 | 7.35 ± 0.03 | 7.40 ± 0.02 |
| Ketone bodies (mmol/L) | 0.5-3 | Low | Normal | Normal |
| Serum iron (µg/dL) | 60-170 | 45 ± 10 | Normal | Normal |
| Hepcidin (ng/mL) | 20-50 | 250 ± 30 | Normal | Normal |
Table 1: Biomarker levels and glycolysis indicators.
Lactate: Lactate levels were markedly elevated in the advanced TNBC group (5.2 ± 0.7 mmol/L), moderately increased in the non-advanced TNBC group (3.8 ± 0.5 mmol/L) and within normal range in the control group (1.5 ± 0.4 mmol/L, p<0.001). Elevated lactate levels indicate enhanced glycolysis in TNBC cells, particularly in advanced cases (Figure 1).
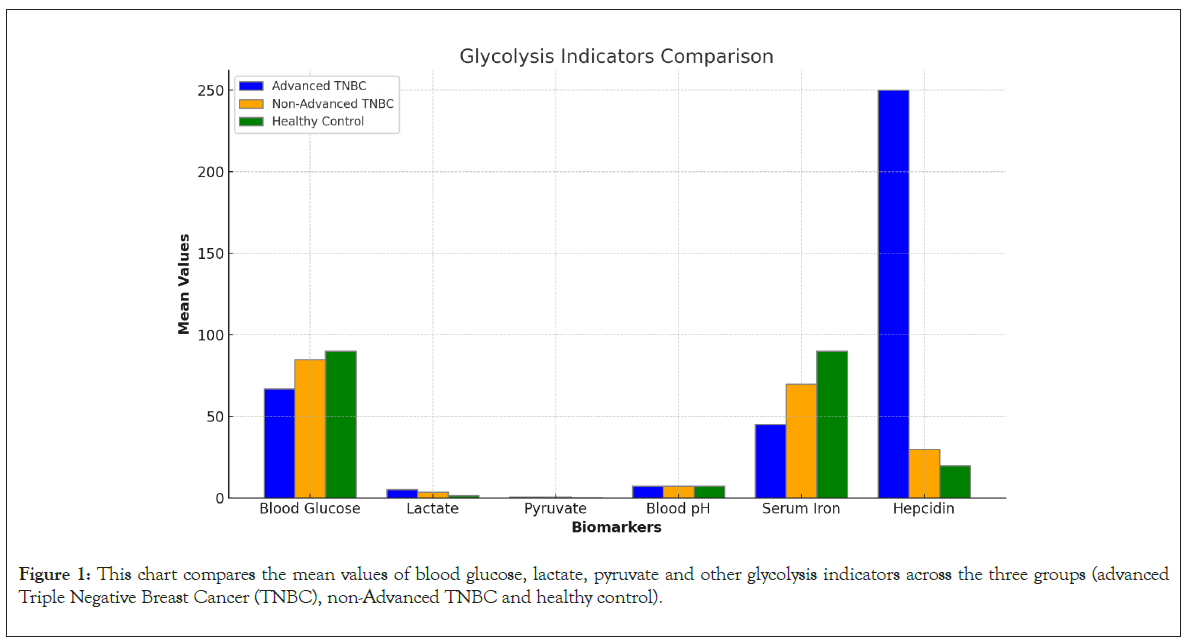
Figure 1: This chart compares the mean values of blood glucose, lactate, pyruvate and other glycolysis indicators across the three groups (advanced Triple Negative Breast Cancer (TNBC), non-Advanced TNBC and healthy control).
Pyruvate: Pyruvate levels followed a similar pattern, being highest in the advanced TNBC group (0.9 ± 0.2 mmol/L) compared to the non-advanced TNBC group (0.7 ± 0.1 mmol/L) and the control group (0.4 ± 0.1 mmol/L, p<0.01). The elevated levels of both lactate and pyruvate underscore the reliance of advanced TNBC on glycolytic pathways.
Insulin and C-peptide: Insulin and C-peptide levels showed significant variability, with some advanced TNBC patients exhibiting hyperinsulinemia, while others, particularly those with cachexia, had lower levels. C-peptide levels followed a similar pattern. This variability may reflect the heterogeneous metabolic demands of TNBC tumors.
Blood pH: Patients with advanced TNBC exhibited mild acidosis (pH 7.28 ± 0.05) compared to the non-advanced TNBC group (pH 7.35 ± 0.03) and the control group (pH 7.40 ± 0.02, p<0.01). The acidification of the blood is likely a consequence of increased lactate production and reduced buffering capacity.
Ketone bodies: Ketone bodies were low in advanced TNBC patients, indicating a reliance on glycolysis over fatty acid oxidation.
Serum iron and hepcidin: Serum iron levels were critically low in the advanced TNBC group (45 ± 10 μg/dL) with correspondingly high hepcidin levels (250 ± 30 ng/mL), reflecting significant iron sequestration and anemia of chronic disease. These findings are consistent with the role of hepcidin in TNBC progression and its potential as a therapeutic target.
Inflammatory markers
IL-6 and TNF-alpha: The levels of inflammatory markers IL-6 and TNF-alpha were significantly elevated in the advanced TNBC group (IL-6: 45 ± 10 pg/mL, TNF-alpha: 35 ± 8 pg/mL) compared to the non-advanced TNBC group (IL-6: 15 ± 5 pg/mL, TNF-alpha: 10 ± 3 pg/mL) and the control group (IL-6: 5 ± 2 pg/mL, TNF-alpha: 3 ± 1 pg/mL, p<0.001). These markers are indicative of a chronic inflammatory state that supports tumor progression and contributes to the poor prognosis associated with advanced TNBC (Table 2 and Figure 2).
| Biomarker | Normal range | Advanced Triple Negative Breast Cancer (TNBC) (TxNxM1G3) |
Non-advanced TNBC (T1N0M0G1) | Healthy control |
|---|---|---|---|---|
| Interleukin-6 (pg/mL) | <5 | 45 ± 10 | 15 ± 5 | 5 ± 2 |
| Tumor necrosis factor-alpha (pg/mL) | <8 | 35 ± 8 | 10 ± 3 | 3 ± 1 |
| Dopamine (pg/mL) | 250-300 | 50 ± 20 | 150 ± 30 | 250 ± 40 |
| Serotonin (ng/mL) | 100-200 | Low | Moderate | Normal |
Table 2: Inflammatory markers and neurotransmitters.
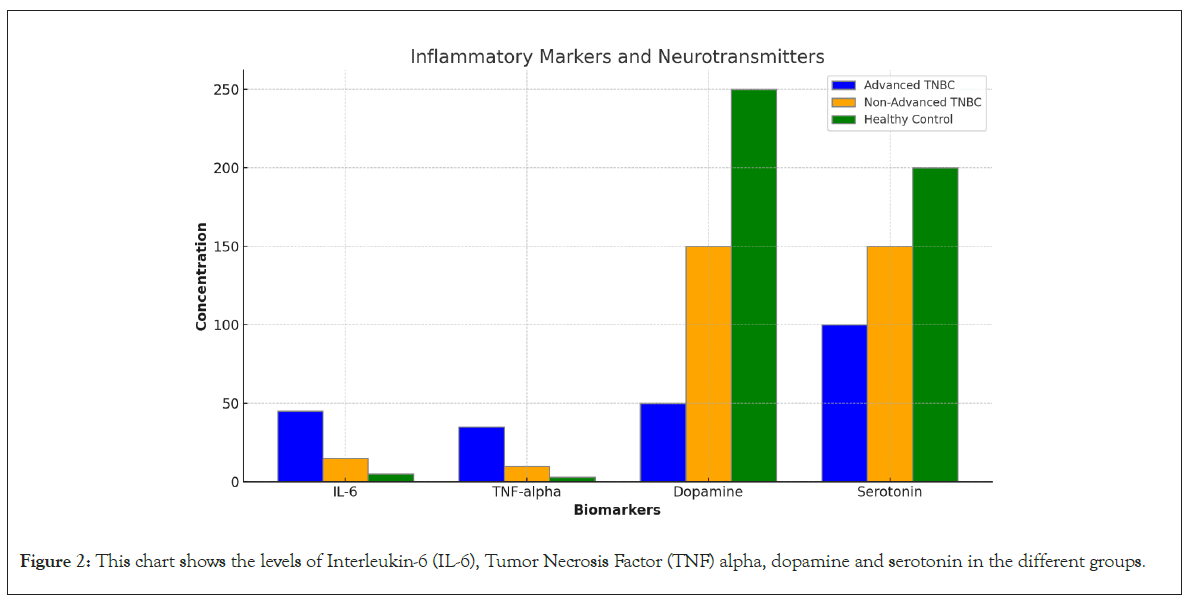
Figure 2: This chart shows the levels of Interleukin-6 (IL-6), Tumor Necrosis Factor (TNF) alpha, dopamine and serotonin in the different groups.
Neurotransmitters
Dopamine: Dopamine levels were critically low in advanced TNBC patients (Blood: 50 ± 20 pg/mL, Urine: 120 ± 30 μg/day) compared to non-advanced TNBC patients (Blood: 150 ± 30 pg/mL, Urine: 200 ± 40 μg/day) and controls (Blood: 250 ± 40 pg/mL, Urine: 300 ± 50 μg/day, p<0.01). This suggests a severe neuroendocrine disruption in advanced TNBC, which may contribute to the observed clinical symptoms and poorer outcomes.
Serotonin: Serotonin levels were also decreased in advanced TNBC patients compared to the other groups, although the decrease was less dramatic than that of dopamine. Reduced serotonin levels could impact mood and immune function, further complicating the clinical management of TNBC.
Matrix Metalloproteinases (MMPs)
MMP-1, MMP-3, MMP-7: These MMPs were moderately elevated in the advanced TNBC group, reflecting increased Extracellular Matrix (ECM) remodeling and tumor invasiveness (Table 3).
| Biomarker | Normal range | Advanced TNBC (TxNxM1G3) | Non-advanced TNBC (T1N0M0G1) | Healthy control |
|---|---|---|---|---|
| MMP-1 (ng/mL) | 1-10 | Elevated | Moderate | Normal |
| MMP-2 (ng/mL) | 10-50 | Markedly elevated | Moderate | Normal |
| MMP-3 (ng/mL) | 1-10 | Elevated | Moderate | Normal |
| MMP-7 (ng/mL) | 1-10 | Elevated | Moderate | Normal |
| MMP-9 (ng/mL) | 10-100 | Markedly elevated | Moderate | Normal |
| Melatonin (pg/mL) | 10-50 | Low | Reduced | Normal |
| Melatonin sulfate (µg/day) | 10-50 | Low | Reduced | Normal |
Note: MMP: Matrix metalloproteinases; TNBC: Triple Negative Breast Cancer.
Table 3: Matrix metalloproteinases and adaptive biomarkers.
MMP-2 and MMP-9: These MMPs were markedly elevated in advanced TNBC, indicating a high degree of ECM degradation and a potential for metastasis. Elevated MMP levels are closely associated with the aggressive behavior of TNBC and poor patient outcomes (Figure 3).
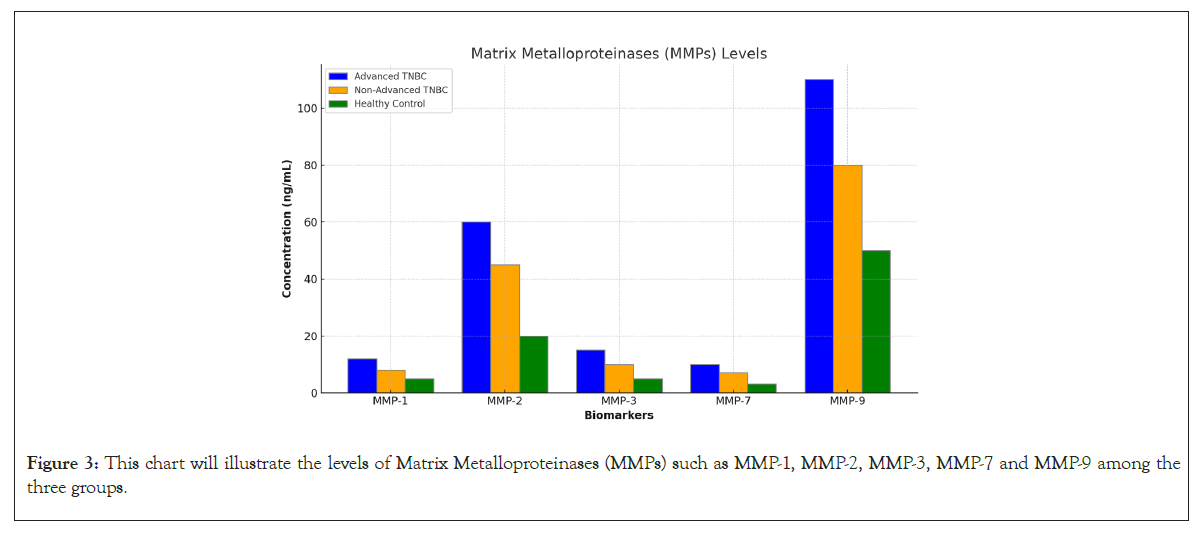
Figure 3: This chart will illustrate the levels of Matrix Metalloproteinases (MMPs) such as MMP-1, MMP-2, MMP-3, MMP-7 and MMP-9 among the three groups.
Adaptive biomarkers
Melatonin and melatonin sulfate: Both melatonin in blood and melatonin sulfate in 24-hour urine were critically low or undetectable in advanced TNBC patients. This contrasts with the non-advanced TNBC group, where levels were reduced but detectable and the control group, which had normal levels. The suppression of these adaptive biomarkers suggests a diminished ability to counteract tumor progression, contributing to the poorer prognosis observed in advanced TNBC (Figure 4).
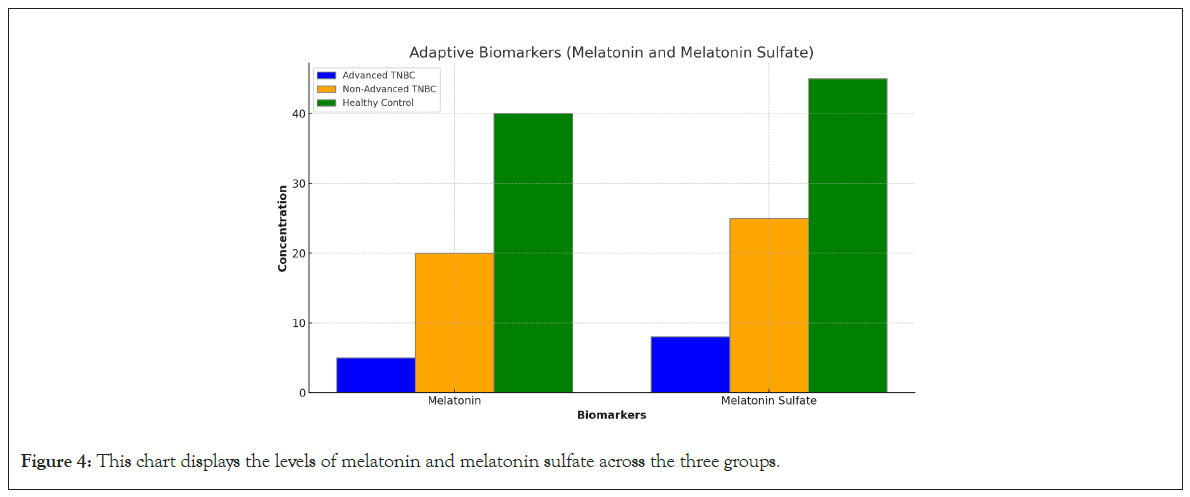
Figure 4: This chart displays the levels of melatonin and melatonin sulfate across the three groups.
Survival analysis
A comparison of survival rates between the 12 patients with irreversible changes in biomarker levels and the rest of the TNBC patients revealed significant differences. The box plot illustrates that the group with irreversible changes had shorter survival times, with a narrower range, indicating a consistently poorer prognosis. In contrast, the other TNBC patients exhibited a wider range of survival times, suggesting more variability but generally better outcomes. This highlights the importance of these biomarkers in predicting survival and guiding treatment strategies (Figures 5 and 6).
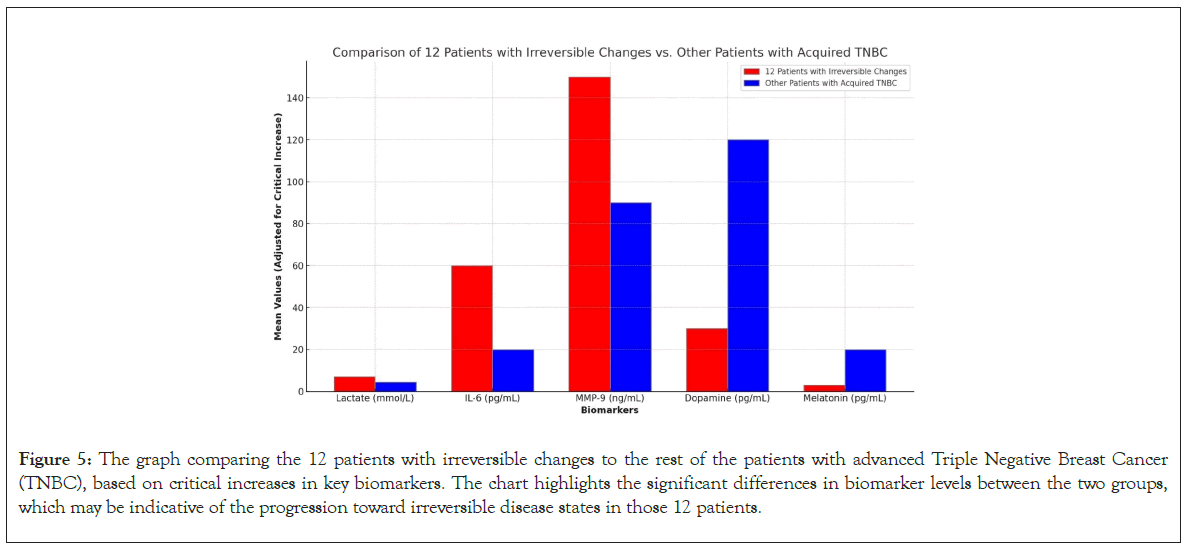
Figure 5: The graph comparing the 12 patients with irreversible changes to the rest of the patients with advanced Triple Negative Breast Cancer (TNBC), based on critical increases in key biomarkers. The chart highlights the significant differences in biomarker levels between the two groups, which may be indicative of the progression toward irreversible disease states in those 12 patients.
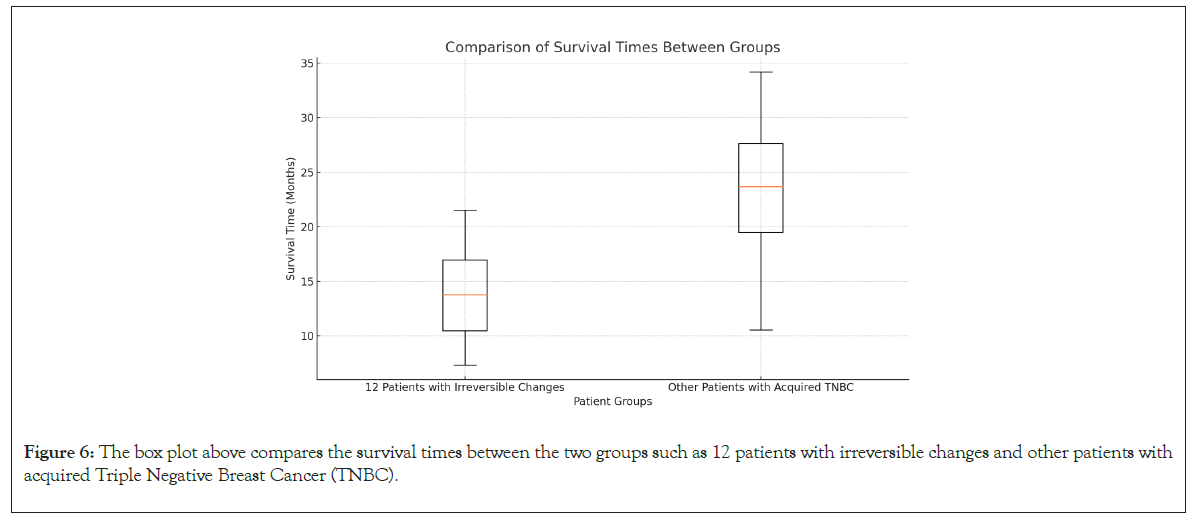
Figure 6: The box plot above compares the survival times between the two groups such as 12 patients with irreversible changes and other patients with acquired Triple Negative Breast Cancer (TNBC).
The results of this study underscore the significant metabolic, inflammatory and neuroendocrine disruptions in patients with advanced TNBC. The elevated glycolysis markers, particularly lactate and pyruvate, indicate a heavy reliance on glycolysis for energy production, characteristic of the Warburg effect. This metabolic reprogramming is associated with increased tumor aggressiveness and a poorer prognosis, as reflected in the survival analysis.
The critically low dopamine and melatonin levels highlight severe neuroendocrine disruption in advanced TNBC, suggesting that these adaptive biomarkers could serve as important indicators of disease progression. The elevated levels of IL-6 and TNF-alpha further emphasize the chronic inflammatory state in TNBC, which not only promotes tumor growth but also contributes to systemic symptoms like cachexia and fatigue [11,12].
The markedly elevated MMP-2 and MMP-9 levels in advanced TNBC patients indicate a high potential for ECM degradation and metastasis, reinforcing the aggressive nature of the disease. These findings suggest that targeting MMPs, along with metabolic and inflammatory pathways, could be a viable therapeutic approach in advanced TNBC.
Hepcidin’s role as a regulator of iron metabolism and its elevated levels in advanced TNBC suggest that it could be a valuable prognostic biomarker and a therapeutic target. The potential combination of hepcidin inhibitors with chemotherapy offers a potential strategy to alleviate anemia, enhance treatment efficacy and improve survival outcomes in TNBC patients, particularly those with advanced disease and irreversible changes in biomarkers.
The next section will provide a comprehensive conclusion, summarizing the study's key findings and discussing potential future research directions and clinical applications.
Implications of low dopamine levels in Triple Negative Breast Cancer (TNBC)
Dopamine is a critical neurotransmitter that plays a role not only in the central nervous system but also in various peripheral processes, including immune function and cellular proliferation. The observation of low dopamine levels in patients with advanced TNBC (TxNxM1G3) has several important implications [13-16].
Immune system dysfunction: It includes immune modulation and chronic inflammation.
(a) Immune modulation: Dopamine has been shown to influence the activity of various immune cells, including T cells, B cells and macrophages. Low dopamine levels can lead to a compromised immune response, making it easier for cancer cells to evade immune surveillance. This may contribute to the progression of TNBC and the development of metastases.
(b) Chronic inflammation: Reduced dopamine may exacerbate the chronic inflammatory state often seen in TNBC patients. Dopamine typically exerts anti-inflammatory effects by modulating the release of pro-inflammatory cytokines like IL-6 and TNFalpha. Low dopamine levels could thus enhance the inflammatory environment, which supports tumor growth and resistance to treatment.
Tumor growth and proliferation: It includes cellular proliferation and angiogenesis.
(a) Cellular proliferation: Dopamine can act as a growth regulator in certain tissues. Low levels of dopamine may remove a natural inhibitory signal on cell proliferation, allowing cancer cells to multiply more rapidly. This is particularly concerning in aggressive cancers like TNBC, where unchecked proliferation leads to rapid tumor growth and early metastasis.
(b) Angiogenesis: Dopamine has anti-angiogenic properties, meaning it can inhibit the formation of new blood vessels that tumors need for growth and metastasis. Low dopamine levels may result in increased angiogenesis, facilitating tumor expansion and the spread of cancer cells to other parts of the body.
Psychological and systemic effects: It includes depression, fatigue and reduced treatment adherence.
(a) Depression and fatigue: Dopamine is heavily involved in the regulation of mood, motivation and energy levels. Low dopamine levels are commonly associated with depression and fatigue, which are prevalent in cancer patients. These symptoms can significantly impact a patient’s quality of life and their ability to adhere to treatment regimens.
(b) Reduced treatment adherence: The psychological effects of low dopamine, such as depression, can lead to reduced motivation to continue with aggressive treatments like chemotherapy. This non-adherence could lead to poorer outcomes and decreased survival rates.
Potential therapeutic targets: It includes dopamine agonists and combination therapies.
(a) Dopamine agonists: The findings suggest that dopamine agonists, which increase dopamine levels, could be explored as a supportive therapy in TNBC. These agents might help modulate the immune response, reduce inflammation and improve patient mood and energy levels, potentially enhancing overall treatment outcomes.
(b) Combination therapies: Combining dopamine-modulating therapies with standard treatments like chemotherapy could be a novel approach. For instance, by boosting dopamine levels, it might be possible to reduce tumor growth and improve the immune system’s ability to fight the cancer, while also alleviating the psychological burden on patients.
Biomarker for disease progression: It includes prognostic marker.
(a) Prognostic marker: Low dopamine levels could serve as a biomarker for more advanced or aggressive TNBC. Monitoring dopamine levels in patients might help identify those at higher risk for poor outcomes, allowing for more personalized and intensive treatment strategies.
Low dopamine levels in TNBC patients have far-reaching implications, affecting everything from immune function and tumor growth to psychological well-being and treatment adherence. Understanding and addressing this neuroendocrine disruption could be key to improving the management of TNBC, potentially leading to better patient outcomes and higher survival rates. Future research should focus on exploring dopamine as both a biomarker and a therapeutic target in TNBC, particularly in advanced stages of the disease [17-20].
Boosting dopamine levels in patients with Triple Negative Breast Cancer (TNBC)
Boosting dopamine levels in patients with Triple Negative Breast Cancer (TNBC) could potentially help address the neuroendocrine disruption, improve mood and energy levels and possibly enhance immune function and treatment adherence. Here are some therapies that might be considered for boosting dopamine in TNBC patients.
Dopamine agonists: These are drugs that mimic the action of dopamine by stimulating dopamine receptors in the brain. These medications are commonly used to treat conditions like Parkinson's disease and restless leg syndrome, but they may also have potential benefits in cancer care. These include pramipexole, ropinirole and bromocriptine.
(a) Pramipexole and ropinirole: These are non-ergot dopamine agonists that are well-tolerated and widely used in neurological conditions. They could be considered for use in TNBC patients to help boost dopamine levels, particularly in those experiencing depression or fatigue.
(b) Bromocriptine: Another dopamine agonist, bromocriptine, has been used to treat hyperprolactinemia and Parkinson's disease. It might also have a role in managing dopamine deficiency in cancer patients, although its use would need to be carefully monitored due to potential side effects.
Monoamine Oxidase Inhibitors (MAOIs): These are a class of antidepressants that work by inhibiting the enzyme monoamine oxidase, which breaks down dopamine in the brain. By inhibiting this enzyme, MAOIs can increase dopamine levels.
(a) Selegiline: This MAOI is often used in the treatment of Parkinson's disease. It selectively inhibits monoamine oxidase B, which specifically breaks down dopamine, making it a potential therapy for increasing dopamine levels in TNBC patients.
(b) Rasagiline: Similar to selegiline, rasagiline is another selective MAO-B inhibitor that could be considered to boost dopamine levels.
Bupropion: It is an atypical antidepressant that acts as a Norepinephrine Dopamine Reuptake Inhibitor (NDRI). It increases the levels of norepinephrine and dopamine in the brain by inhibiting their reuptake.
(a) Use in TNBC: Bupropion has been used off-label for managing depression in cancer patients and could help alleviate depressive symptoms in TNBC patients by increasing dopamine levels. It is also associated with fewer sexual side effects compared to other antidepressants, making it a favorable option for some patients.
Levodopa (L-DOPA): It is a precursor to dopamine and is commonly used in the treatment of Parkinson's disease. It crosses the blood-brain barrier and is converted into dopamine in the brain. While primarily used in neurological conditions, L-DOPA could theoretically be used to increase dopamine levels in TNBC patients. However, it requires careful dosing and monitoring due to potential side effects, such as dyskinesias and fluctuations in efficacy.
Nutritional and lifestyle interventions: Diet and supplements can also play a role in boosting dopamine levels, particularly in conjunction with medical therapies.
(a) Tyrosine-rich foods: Tyrosine is an amino acid that is a precursor to dopamine. Consuming foods high in tyrosine, such as lean proteins (chicken, turkey, fish), eggs, dairy, nuts and beans, may support dopamine synthesis.
(b) Omega-3 fatty acids: Omega-3s have been shown to support brain health and neurotransmitter function, potentially aiding in the production of dopamine.
(c) Exercise: Regular physical activity, particularly aerobic exercise, has been shown to increase dopamine levels and improve mood and cognitive function. Encouraging TNBC patients to engage in regular exercise could be beneficial for boosting dopamine.
Behavioral and psychological therapies: Cognitive Behavioral Therapy (CBT) and other psychological interventions can help improve mood and manage symptoms of depression and anxiety, which are linked to dopamine dysregulation. This therapy can help patients develop strategies to cope with cancer-related stress and fatigue, indirectly supporting dopamine regulation by improving mental health and resilience.
Experimental approaches: Emerging therapies that modulate dopamine levels are being investigated in various clinical settings and may offer future avenues for treatment in TNBC.
(a) Gene therapy: Research is ongoing into gene therapy approaches that could increase dopamine production or enhance dopamine receptor function. While still experimental, such therapies could eventually offer a targeted way to correct dopamine deficiencies in cancer patients.
(b) Neurostimulation techniques: Techniques like Transcranial Magnetic Stimulation (TMS) and Deep Brain Stimulation (DBS) are being explored for their potential to enhance dopamine release and receptor sensitivity. These approaches might be applicable in cases of severe neuroendocrine disruption, although their use in cancer patients would require further research.
Boosting dopamine levels in TNBC patients through pharmacological, nutritional, lifestyle and psychological interventions could help manage symptoms associated with dopamine deficiency, such as depression, fatigue and possibly immune dysfunction. While some of these therapies are established in other medical fields, their application in TNBC would require careful consideration and monitoring. Clinical trials and further research are needed to explore the safety and efficacy of these approaches in the context of TNBC treatment [21-25].
Combination therapies
In addition to combining hepcidin inhibitors with chemotherapy, several other treatment combinations could potentially enhance the efficacy of therapies for Triple Negative Breast Cancer (TNBC). Here are some possible treatment combinations.
Chemotherapy+immunotherapy: TNBC is often characterized by high levels of immune evasion. Immune checkpoint inhibitors, such as Programmed Cell Death 1 (PD-1)/Programmed Cell Death Ligand 1 (PD-L1) or Cytotoxic T-Lymphocyte Associated protein 4 (CTLA-4) inhibitors, can enhance the body’s immune response against cancer cells. Combining these with chemotherapy could help prime the immune system by releasing tumor antigens, making immunotherapy more effective. For example, pembrolizumab (a PD-1 inhibitor) combined with chemotherapy has shown promise in TNBC, particularly in patients with PD-L1 positive tumors.
Chemotherapy+PARP inhibitors: TNBC often exhibits defects in Deoxyribonucleic Acid (DNA) repair mechanisms, particularly in patients with Breast Cancer gene 1 (BRCA1) or Breast Cancer gene 2 (BRCA2) mutations. Poly (ADP-ribose) Polymerase (PARP) inhibitors exploit these defects by further hindering DNA repair, leading to cancer cell death. Combining PARP inhibitors with chemotherapy can enhance this effect. For example, olaparib or talazoparib combined with platinum-based chemotherapy in patients with BRCA-mutated TNBC.
Chemotherapy+anti-angiogenic therapy: Tumors require new blood vessels to grow and metastasize. Anti-angiogenic agents, such as bevacizumab, inhibit Vascular Endothelial Growth Factor (VEGF), thereby disrupting the tumor’s blood supply. Combining these agents with chemotherapy can enhance tumor response and prolong progression-free survival. For example, bevacizumab combined with paclitaxel in TNBC patients has shown some benefit, particularly in advanced cases.
Targeted therapy+chemotherapy: It includes Androgen Receptor (AR) inhibitors and Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (PKB)/mammalian Target of Rapamycin (mTOR) inhibitors.
(a) Androgen Receptor (AR) inhibitors: A subset of TNBC expresses androgen receptors. Targeting these receptors with AR inhibitors, such as enzalutamide, in combination with chemotherapy might provide a novel approach for treating ARpositive TNBC. For example, enzalutamide combined with taxane chemotherapy in AR-positive TNBC.
(b) PI3K/PKB/mTOR inhibitors:The PI3K/PKB/mTOR pathway is often activated in TNBC, promoting tumor growth and survival. Inhibitors of this pathway can be combined with chemotherapy to improve outcomes. For example, alpelisib (a PI3K inhibitor) or everolimus (an mTOR inhibitor) combined with chemotherapy.
Chemotherapy+epigenetic therapy: Epigenetic modifications can lead to the silencing of tumor suppressor genes. Histone Deacetylase (HDAC) inhibitors can reverse these modifications, potentially sensitizing TNBC cells to chemotherapy. For example, vorinostat or entinostat combined with chemotherapy in TNBC.
Chemotherapy+hormonal therapy (for AR-positive TNBC): In AR-positive TNBC, combining anti-androgens with chemotherapy might offer a treatment option, particularly in hormone receptorindependent pathways. For example, bicalutamide combined with chemotherapy in AR-positive TNBC.
Targeted therapy+immunotherapy: Combining PARP inhibitors with immune checkpoint inhibitors could exploit the DNA damage response to enhance anti-tumor immunity. For example, olaparib combined with pembrolizumab in BRCA-mutated TNBC.
Radiotherapy+immunotherapy: Radiation therapy can cause immunogenic cell death, which can be enhanced by combining it with immunotherapy. This combination can potentially turn the tumor into an in-situ vaccine. For example, radiotherapy combined with nivolumab (PD-1 inhibitor).
Chemotherapy+metformin: Originally used to treat type 2 diabetes, metformin has shown potential anti-cancer effects, particularly by inhibiting the mTOR pathway and reducing insulin levels, which can influence cancer cell growth. Combining metformin with chemotherapy could enhance the anti-cancer effects. For example, metformin combined with doxorubicin or other chemotherapeutic agents.
Nutritional and metabolic therapies+standard treatment: A ketogenic diet, which is low in carbohydrates and high in fats, could theoretically enhance the effectiveness of chemotherapy by lowering glucose availability to cancer cells that rely on glycolysis. This diet could be used in conjunction with standard treatments. For example, ketogenic diet combined with chemotherapy in patients with TNBC.
For the summarize, the combination of various therapies with chemotherapy in TNBC offers multiple avenues to potentially improve patient outcomes. Each of these combinations targets different aspects of tumor biology, from DNA repair and immune evasion to angiogenesis and metabolism. The choice of combination should be customized to the patient's specific tumor characteristics, such as BRCA mutation status, androgen receptor expression or the presence of specific molecular pathways like PI3K/PKB/mTOR [26-29].
A holistic treatment guideline for Triple Negative Breast Cancer (TNBC) in patients with overproduction of MMP-2 and MMP-9 based on the principles of traditional Chinese medicine
Triple Negative Breast Cancer (TNBC) is a particularly aggressive and difficult-to-treat subtype of breast cancer, characterized by the absence of estrogen receptors, progesterone receptors and HER2 amplification. One of the distinctive feature of TNBC is the overproduction of Matrix Metalloproteinases (MMPs), particularly MMP-2 and MMP-9, which contribute to the degradation of the extracellular matrix, facilitating tumor invasion and metastasis. Given the limited efficacy of conventional treatments for TNBC, there is growing interest in integrating Traditional Chinese Medicine (TCM) principles with modern oncology to create a holistic treatment approach [30].
This guideline explores a comprehensive treatment strategy for TNBC patients with overproduction of MMP-2 and MMP-9, based on TCM principles and the use of natural products known to reduce the expression of these MMPs.
Principles of Traditional Chinese Medicine (TCM) in cancer treatment
TCM views the human body as an integrated whole, with a focus on balancing the flow of Qi (vital energy) and ensuring harmony among the body's systems. In the context of cancer treatment, TCM aims to strengthen the body's defenses (immune system), reduce toxins and restore balance to bodily functions. The following TCM principles are particularly relevant to the treatment of TNBC [31,32].
Strengthening Zheng Qi (vital energy): Boosting the body's natural resistance to disease.
Eliminating toxins: Removing pathological factors, including those contributing to cancer growth.
Regulating Qi and blood: Ensuring proper circulation and the removal of stasis that may contribute to tumor growth.
Balancing yin and yang: Maintaining the balance of the body’s essential energies to prevent the progression of disease.
Natural products that reduce MMP-2 and MMP-9 expression
Several natural products have been identified for their ability to modulate the expression of MMP-2 and MMP-9, making them potentially valuable in the holistic treatment of TNBC.
Curcumin (turmeric): Curcumin, a polyphenol derived from turmeric, has been shown to inhibit the expression of MMP-2 and MMP-9 through the suppression of the Nuclear Factor Kappa-light (NF-κB) chain enhancer of activated B cells signaling pathway. Turmeric is used in TCM to invigorate the blood, move Qi and alleviate pain. It is particularly valued for its anti-inflammatory and detoxifying properties.
Epigallocatechin Gallate (EGCG) from green tea: EGCG, a catechin found in green tea, downregulates MMP-2 and MMP-9 by inhibiting the activation of transcription factors such as AP-1 and NF-κB. Green tea is known for its cooling properties, helping to clear heat and toxins from the body and is often used to reduce inflammation and support liver function.
Resveratrol: Resveratrol, a polyphenol found in grapes and red wine, has been demonstrated to suppress MMP-2 and MMP-9 expression through inhibition of the PI3K/PKB and Mitogen-Activated Protein Kinases (MAPK)/ Extracellular Signal-regulated Kinase (ERK) signaling pathways. While not traditionally used in TCM, resveratrol's properties align with the principles of clearing heat and detoxifying the body.
Baicalein (from Scutellaria baicalensis): Baicalein, a flavonoid from the roots of Scutellaria baicalensis (Huang Qin), inhibits MMP-2 and MMP-9 expression through the suppression of the NF-κB pathway. Huang Qin is widely used in TCM to clear heat, dry dampness and detoxify, making it an excellent choice for combating cancer-related inflammation.
Ginsenoside Rg3 (from Ginseng): Ginsenoside Rg3, a compound found in Ginseng, inhibits MMP-2 and MMP-9 by modulating the expression of VEGF and suppressing the PI3K/PKB pathway. Ginseng is revered in TCM for its ability to tonify Qi, nourish blood and support the body's overall resilience, particularly during cancer treatment.
Quercetin: Quercetin, a flavonoid found in many fruits and vegetables, reduces MMP-2 and MMP-9 expression by inhibiting the NF-κB and AP-1 pathways. Quercetin's ability to reduce inflammation and promote circulation aligns with TCM's goals of detoxification and balancing Qi and blood.
Holistic treatment approach
The following treatment protocol integrates TCM principles with modern findings on natural products that modulate MMP-2 and MMP-9 expression.
Dietary and herbal therapy: Incorporate natural products like turmeric, green tea, grapes (resveratrol) and Ginseng into the diet. Use TCM herbal formulas that include Scutellaria baicalensis (Huang Qin) and other anti-inflammatory, detoxifying herbs.
Acupuncture: Use acupuncture to regulate Qi and blood, enhance immune function and reduce cancer-related fatigue. Points such as ST36 (Zusanli) and SP6 (Sanyinjiao) may be used to tonify Qi and support overall health.
Qi Gong and Tai Chi: Incorporate mind-body practices like Qi Gong and Tai Chi to enhance the flow of Qi, reduce stress and improve immune function, which may help modulate the expression of MMPs and support the body’s ability to fight cancer.
Conventional cancer therapies: Combine the above TCM approaches with standard treatments such as surgery, chemotherapy and radiation. Natural products that inhibit MMPs can be used adjunctively to reduce tumor invasiveness and metastasis.
Monitoring and adjustment: Regularly monitor the patient’s MMP-2 and MMP-9 levels and adjust the treatment protocol as needed, incorporating more aggressive TCM interventions or modifying the use of natural products based on the patient’s response.
A holistic treatment approach for TNBC that integrates traditional Chinese medicine with modern oncology principles offers a potential pathway for managing the overproduction of MMP-2 and MMP-9. By using natural products that reduce MMP expression, combined with TCM practices that support the body’s overall health and resilience, patients may experience improved outcomes and quality of life. This integrated approach emphasizes the importance of treating the whole person, not just the disease, aligning with the core principles of TCM. Future research and clinical trials are needed to further validate these strategies and optimize treatment protocols for TNBC patients [33].
This study explored the roles of various biomarkers in Triple Negative Breast Cancer (TNBC), with a focus on identifying prognostic and predictive indicators of disease progression. By comparing glycolysis indicators, inflammatory markers, neurotransmitters, Matrix Metalloproteinases (MMPs) and adaptive biomarkers across patients with advanced TNBC, non-advanced TNBC and healthy controls, we have provided valuable insights into the biological underpinnings of this aggressive cancer subtype.
The findings highlighted significant metabolic reprogramming in TNBC, characterized by elevated glycolysis markers such as lactate and pyruvate, which are consistent with the Warburg effect. This metabolic shift, along with low blood glucose and altered pH levels, underscores the aggressive nature of TNBC and its reliance on glycolysis for energy production. Additionally, the elevated levels of inflammatory markers IL-6 and TNF-alpha in advanced TNBC suggest a chronic inflammatory state that supports tumor growth and resistance to treatment.
Neurotransmitter disruptions, particularly low dopamine and serotonin levels, were observed in advanced TNBC patients, indicating severe neuroendocrine dysfunction. These alterations are associated with poorer patient outcomes, including reduced survival rates and highlight the potential of dopamine and serotonin as biomarkers for disease progression and therapeutic targets.
The marked elevation of MMP-2 and MMP-9 in advanced TNBC points to significant extracellular matrix degradation and a high potential for metastasis. These findings emphasize the importance of MMPs in TNBC progression and suggest that targeting MMPs could be a viable therapeutic strategy.
The study also shed light on the role of hepcidin, a key regulator of iron metabolism, in TNBC. Elevated hepcidin levels were associated with anemia of chronic disease, which is commonly observed in TNBC patients. The potential use of hepcidin inhibitors, in combination with chemotherapy, offers a promising approach to alleviate anemia, enhance treatment efficacy and improve overall survival outcomes in TNBC patients.
Our survival analysis further underscored the prognostic significance of these biomarkers. Patients with critical increases in these markers, particularly those with irreversible changes, exhibited significantly shorter survival times compared to other TNBC patients. This highlights the need for more aggressive and targeted therapeutic interventions in this high-risk group.
In conclusion, this study provides a comprehensive analysis of key biomarkers in TNBC and their implications for patient prognosis and treatment. The integration of these biomarkers into clinical practice could lead to more personalized and effective treatment strategies, ultimately improving outcomes for patients with this challenging breast cancer subtype. Future research should focus on validating these biomarkers in larger cohorts and exploring novel therapeutic approaches, such as the combination of hepcidin inhibitors with chemotherapy, to address the multifaceted challenges of treating TNBC.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Tavartkiladze A, Simonia G, Revazishvili P, Maisuradze M, Kasradze D, Tavartkiladze G, et al. (2024). Prognostic and Predictive Biomarkers in Triple Negative Breast Cancer (TNBC): A Comparative Study of Glycolysis, Inflammatory Markers, Melatonin, Neurotransmitters and Metalloproteinases. J Clin Cell Immunol. 15:725.
Received: 21-Oct-2024, Manuscript No. JCCI-24-33592; Editor assigned: 23-Oct-2024, Pre QC No. JCCI-24-33592 (PQ); Reviewed: 07-Nov-2024, QC No. JCCI-24-33592; Revised: 14-Nov-2024, Manuscript No. JCCI-24-33592 (R); Published: 21-Nov-2024 , DOI: 10.35248/2155-9899.24.15.747
Copyright: © 2024 Tavartkiladze A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.