Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Review Article - (2008) Volume 1, Issue 3
Keywords: Epidemiology, BRCA1, BRCA2, Adjuvant hormonal therapy, Proteomics, SELDI-TOF MS
MBC: Male Breast Cancer
LOH: Loss of Heterozygosity
FBC:Female Breast Cancer
EGFR: Epidermal Growth Factor Receptor
FISH: Fluorescence In Situ Hybridization
KIPs: Kinase Inhibitor Proteins
AR: Androgen Receptor
CAG:Polymorphic Polyglutamine
GGC:Polyglycine
ER: Estrogen Receptor
PR: Progesterone Receptor
LCM: Laser Capture Microdissection
2-DE: 2-Dimensional gel Electrophoresis.
MALDI-TOF - MS:Matrix-Assisted Laser Desorption and Ionization with time-Of-Flight Detection Mass Spectrometry
SELDI-TOF MS :Surface-Enhanced Laser Desorption and Ionization with time-Of-Flight Mass Spectrometry
NAF: Nipple Aspirate Fluid
Breast cancer is a malignant tumor that has developed from cells of the breast. It is one of the oldest known forms of cancer tumors. The disease occurs primarily in women but occasionally occurs in men because the breast is composed of identical tissues in males and females. The male breast is primarily composed of stroma and ducts. Lobules are typically absent from the male breast; therefore lobular carcinoma is rarer in men than in women (Koc et al., 2001; Michaels et al., 1999). Almost all the histologic subtypes of breast cancer that have been described in women have also been reported in men. Male breast cancer (MBC) is mainly classified as benign, in situ or invasive.
Breast cancer in men accounts for less than 1% of all breast cancers in the United States and less than 1% of all diagnosed breast cancers (Smolin and Massie, 2002). MBC has been an uncommon disease but now since its incidence is rising (Carmalt et al., 1998); there has been an increasing interest in this disease. The molecular events that underlie neoplastic progression are complex and diverse, and remain incompletely characterized. Researchers are making great progress in understanding how certain changes in DNA can cause normal cells to become cancerous. The DNA damage resulting from mutations, ionizing radiation, carcinogens, stress, loss of heterozygosity (LOH) causing alterations in various genes viz. DNA repair genes, breast cancer susceptibility genes, apoptotic genes and cell growth regulatory genes, is one of the most leading factors for MBC. Besides the above mentioned genes, polymorphism/mutation in some other genes is also some of the causative factors for MBC. Over the last decade, our increasing knowledge of these underlying genes and their proteins, that are associated with the development and progression of MBC, has provided us opportunities to develop targeted therapeutics that have their ideal aim for the disease treatment. The identification, quantification, classification and functional assignment of protein product of the underlying genes will be essential to the full understanding of the molecular events behind the disease. Proteomic studies offer the potential to revolutionize breast cancer prevention and treatment (Bertucci et al., 2006). This review will explore latest information on the epidemiology, risk factors, genes involved, causes, treatment, proteomics approaches and their application in MBC research.
Epidemiology and Risk Factors
Incidence of breast cancer in men is increasing at an alarming rate. In 2007, about 2,030 new cases of MBC have been diagnosed so far and about 450 males have died from breast cancer in the United States (ACS, 2007). Aging is an important risk factor for the development of breast cancer in men. The incidence of disease increases with advancing patient age. The mean age at diagnosis for men with breast cancer is 67 years, which is 5 years older than the average age at diagnosis for women (Giordano et al., 2004). Similar to female breast cancer (FBC), a positive family history in approximately 30% of breast cancer cases was associated with the increased risk of MBC. Breast cancer risk is increased if other members of the family (blood relatives) have had breast cancer (Demeter et al., 1999; Everson et al., 1976). A population-based series of 54 MBC cases observed reported that 17% of MBC patients have at least one first-degree relative with breast cancer (Friedman et al., 1997).
Klinefelter's Syndrome, is a congenital condition in which men have more than one X chromosome, affects about 1 of 1000 men. People suffering from Klinefelter's Syndrome have been shown to be more susceptible towards breast cancer. (Hultborn et al., 1997) found that 7.5% of 93 male breast carcinoma cases from western Sweden were affected by Klinefelter syndrome. As compared with other men, they have lower levels of androgens and more estrogens. For this reason, they often develop gynecomastia which is a benign enlargement of the male breast resulting from a proliferation of the glandular component of the breast. Gynecomastia has been reported to occur in association with breast cancer in men (Kozak et al., 1986; Branstein, 1993). Ionizing radiations have also been recognized as one of the causative factor of breast cancer. A close exposure of chest area to radiation increases the risk of a developing MBC. Since exposure of the breast to ionizing radiation increases risk of cancer in women, the situation may be similar in men exposed to therapeutic or diagnostic radiation (Hsing et al., 1998). There are also studies, which reveal the increased risk of this disease induced by occupational and environmental exposure to pesticides, aromatic hydrocarbons and ionizing radiation (Martynowicz et al., 2005). Heavy alcohol intake, liver diseases such as cirrhosis, obesity, diet have all been proposed as risk factors, but findings are needed to elucidate their role (Lenfant-Pejovic et al., 1990; Sorensen et al., 1998; Weiderpass et al., 2001; Ewertz et al., 2001).
Molecular Epidemiology
Advances in the study of functions of the DNA repair genes, breast cancer susceptibility genes, apoptotic genes and cell growth regulatory genes indicate that their products play important roles in DNA damage repair and chromosome stability. DNA damage can be induced by various DNA damaging agents, such as mutations, carcinogens, LOH, stress or ionizing radiations. When a tumor suppressor gene or protoncogene is mutated, either through loss of a normal tumor suppressor gene or through a gain in function of a proto-oncogene, cell growth may be promoted. Accumulating evidence indicates that breast cancer tumorigenesis is co-related to DNA damage initiated chromosome instability (Ralhan et al., 2007). Impaired DNA repair may elevate the risk of malignant transformation of breast cells due to the accumulation of spontaneous mutations in target genes (Table 1, Figure 1) and increasing susceptibility to exogenous carcinogens. The target genes can be categorized as follows-
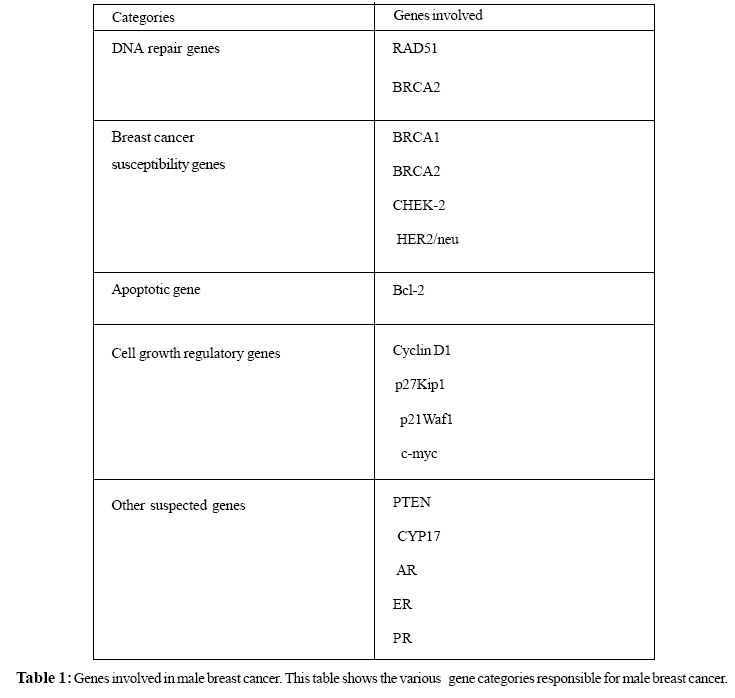
DNA Repair Genes
DNA repair genes encode for enzymes that restore the integrity of the DNA if it is damaged by radiation or carcinogens, or if mismatches occur during the replication process. Mutations in DNA repair genes which lead to a loss in function may accelerate the carcinogenic process by allowing mutations to accumulate. Although, many genes are involved in DNA repair mechanism but BRCA2 and RAD51 are the two specific genes which are reported to be associated with MBC. Men who carry a germ-line BRCA2 mutation have an increased risk of breast cancer (Karhu et al., 2006; James Brenner et al., 2004). BRCA2 gene found to be associated with RAD 51 gene is involved in DNA repair mechanism. The formation of RAD51 foci in response to DNA damage is dependent on BRCA2 and a series of proteins known as the RAD51 paralogs (RAD51B, RAD51C, RAD51D, XRCC2 and XRCC3) which forms a multiprotein complex that forming a heterodimer with each of the gene products playing a different role in DNA repair by homologous recombination (Schild et al., 2000). Some of these genes have already been proposed as breast cancer susceptibility gene (Rodriguez-Lopez et al., 2004).
Breast Cancer Susceptibility Genes
The protein products of the breast cancer susceptibility genes BRCA1, BRCA2 and p53, CHEK-2 and HER2/ neu are all components of the molecular pathways accelerated in response to impaired DNA damage repair (Franke et al., 2006). Germline mutation in breast cancer susceptibility genes BRCA1, BRCA2 are most commonly responsible for developing approximately 80-90% of hereditary breast cancer, whereas they are not very frequent in sporadic breast cancers (de Jong et al., 2002). In women, both BRCA1 and BRCA2 are thought to account for most hereditary breast cancers. BRCA1 (a tumor suppressor gene) mutation has been reported in men with breast cancer, although they do not appear to be common cause of MBC (Sverdlov et al., 2000; Struewing et al., 1995; Basham et al., 2002; Friedman et al., 1997; Ottini et al., 2003). However, mutations in the BRCA2 gene are more frequent in males with breast cancer. This gene was first identified by Wooster, who localized it to 13q12-13 and described multiple linkages to this area (Wooster et al., 1994). 4%-16% of men with breast cancer were reported to be BRCA2 mutation carriers in population-based series (Mlika-Cabanne et al., 2002; Friedman et al., 1997; Ottini et al., 2003; Wooster et al., 1994). LOH in BRCA2 gene has been demonstrated to play an important role in the development of BRCA2 induced breast cancer (Eiriksdottir et al., 1998). In a population- based study in Italy, LOH at one or more of nine loci in the BRCA2 region were found in 8 of 22 tumors tested (Ottini et al., 2003;Wooster et al., 1994; Eiriksdottir et al., 1998; Sigbjornsdottir et al., 2000).
Apoptotic Genes
Apoptosis is the normal physiological response to many stimuli, including irreparable DNA damage. Normal breast development is controlled by a balance between cell proliferation and apoptosis, and there is strong evidence that tumor growth is not just a result of uncontrolled proliferation but also of reduced apoptosis. The balance between proliferation and apoptosis is crucial in determining the overall growth or regression of the tumor (Reed, 1999; Tamm et al., 2001). Bcl-2 is the founder of a large family of apoptosis regulators that either promote cell survival or facilitate cell death. In breast cancer in women, expression of Bcl-2 has been associated with favorable prognostic features (Joensuu et al., 1994). Several studies have investigated the incidence of Bcl-2 expression in breast cancer in men. Overall, Bcl-2 expression was seen in 153 (79%) of 193 cases (Weber-Chappuis et al., 1996; Pich et al., 2000; Pich et al.,1998). Men may have significantly higher rates of Bcl-2 expression than women (Weber-Chappuis et al., 1996). The high rates of expression of Bcl-2 in male breast cancer suggest that apoptotic mechanisms may be important in the etiology of breast cancer in men.
Cell Growth Regulatory Genes
The cell cycle is governed by a family of Cdks. Cyclins are prime cell cycle regulators and play a central role in the control of cell proliferation by forming a complex with different Cdks . Abnormalities in cell cycle regulators due to various factors including mutations, stress etc and subsequent deregulation of the G1-S transition may be one of the most important biological events in malignant cell transformation. Cyclin D1 is involved in cell-cycle regulation and helps control the cell's entry into S phase. In breast cancer in women, this gene is oncogenic but appears to be associated with a favorable prognosis (Dickerson and Lippman, 2000). A total of 117 tumors of the male breast were tested for cyclin D1 overexpression; 60 (51%) were immunoreactive (Rayson et al., 1998; Arber et al., 1995). This number is very similar to the 50% rate of overexpression seen in women (Dickerson and Lippman, 2000). Rayson and colleagues, (1998)found that cyclin D1 negativity was associated with significantly decreased progression-free survival, indicating that gene overexpression may be a favorable prognostic factor in men with carcinoma of the breast.
Kinase inhibitor proteins (KIPs) p27Kip1 and p21Waf1 negatively regulate cell cycle progression by preventing the passage of cycling cells from G1 to S phase through G1 cyclin-dependent kinase activation. p27Kip1 is a cdk inhibitor , which has been shown to inhibit the activity of cyclin A-cdk2, cyclin B-cdk2, cyclin D-cdk4 and cyclin E-cdk2 by preventing cdk activation and thereby precluding cells from entering S phase. In one of the study by Curigliano et al., (2002) the immunohistochemical expression of p21Waf1 and p27Kip1 protein in 27 primary MBC and in 101 FBC was seen and it was found that p27Kip1 and p21Waf1 immunoreactivity was higher in MBCs compared with FBCs. The findings of higher p27Kip1 and p21Waf1 immunostaining may be an additional predictive factor in MBC.
Overexpression of c-myc gene may be regarded as an additional prognostic factor in MBC. Most, if not all, types of human malignancy have been reported to have amplification and/or overexpression of this gene. Aberrant expression of c-myc has also been consequently cause apoptosis. Studies in recent years have further shown that the c-myc gene regulates growth, both in the sense of cell size and in the context of tissue differentiation (Gandarillas and Watt, 1997; Iritani and Eisenman, 1999; Johnston et al., 1999; Schmidt, 1999; Schuhmacher et al., 1999). Thus, it is now known that the c-myc gene participates in most aspects of cellular function, including replication, growth, metabolism, differentiation, and apoptosis in response to extracellular signals (Packham and Cleveland, 1998; Hoffman and Liebermann, 1998; Dang, 1999; Dang et al., 1999; Elend and Eilers, 1999; Prendergast, 1999). Among the putative c-myc target genes (cyclins D1, D2, E and A, cdk4, e2f1, e2f 2, cdc25A and B, etc.) only cdk4, e2f 2 and cyclins D1 and D2 seem to encompass a Myc E-box element in their regulatory regions (Liao and Dickson, 2000). To induce a tumor, c-myc may need not only to promote cell proliferation but also simultaneously to inhibit its tendency for cell death, so as to increase the cell number to form a tumor mass. Therefore, the role of c-myc in inhibiting apoptosis is easily connected to its tumorigenicity. Out of 50 patients with MBC, 82% of cases was found to be immunopositive for c-myc, a rate far higher than that detected by gene amplification in FBC (4% to 41%). c-myc expression was associated with a shorter survival univariate analysis; this is the first report of this association in MBC (Pich et al., 2000). However, additional studies confirming these results in larger populations are needed.
Involvement of Other Suspected Genes
Polymorphism/mutations in other genes has been reported that are responsible for causing breast cancer in men includes PTEN (Cowden’s syndrome), CYP17, androgen receptor (AR) gene, estrogen receptor (ER) gene, and progesterone receptor (PR) gene. Cowden syndrome is an autosomal dominant cancer susceptibility syndrome characterized by multiple hamartomas and is associated with germ line mutations in the PTEN tumor suppressor gene (Fackenthal et al., 2001). Germ line PTEN mutations contribute to the development of both MBC and FBC within Cowden syndrome families and may be associated with earlier onset of cancer (Fackenthal et al., 2001).Two cases of MBC in Cowden syndrome families have been reported, both with early onset and confirmed germline PTEN mutations (Thorlacius et al., 1997). PTEN mutations do not; however, seem to be a major cause of breast cancer in men without phenotypic abnormalities of Cowden syndrome. The CYP17 gene codes for the cytochrome P450c17- enzyme responsible for steroid 17-hydroxylation and 17, 20-lyase activity, and is a key regulator of steroid synthesis. A polymorphic T to C substitution creates an additional promoter site that increases gene transcription and steroid synthesis (Carey et al., 1994). In a case-control study of 64 men with breast cancer and 81 controls, a C allele of the CYP17 gene was present more frequently in the patients, with an odds ratio of 2·1 (Young et al.,1999). A Scottish case control study (Young et al., 1999) examined the association between CYP17 polymorphisms and MBC. Results from this study of 76 MBC patients indicated that the CYP17 variant allele was found more frequently in breast cancer patients than in controls. The variant genotype associated with higher serum estrogen levels, however, has not been consistently associated with FBC (Feigelson et al., 1998; Dunning et al., 1998). Given that natural levels of estrogen are higher in females than males,Young et al. (1999) hypothesized that, in females, any increases might not have a significant effect on breast cancer development beyond already high levels. Correspondingly, Young et al. (1999) reasoned that the association between serum estrogen and breast cancer may be detectable only in men. In an Icelandic study of 39 male patients, 15 of whom had a BRCA2 mutation, a statistically non-significant increase in frequency of the CC genotype was found in carriers of 999del5 mutation (33% vs 17%) (Gudmundsdottir et al., 2003).These studies suggest, but do not prove, a link between CYP17 and risk of MBC in both BRCA2 carriers and non-carriers.
Germ line mutations in the androgen receptor (AR) gene have also been suggested to predispose to MBC (Lobaccaro et al., 1993). Wooster et al., (1992) first reported an association between development of breast cancer in two brothers and a germ line mutation in exon 3 encoding the DNA-binding domain of the AR. The AR gene has highly polymorphic polyglutamine (CAG) and polyglycine (GGC) tracts within the coding area of exon 1. Long CAG repeats have been implicated in FBC most likely by decreasing the capacity of the receptor to activate transcription (Giguere et al., 2001). CAG repeats among MBC patients have been examined in three studies, and no statistically significant association between CAG repeat length and MBC were observed (Friedman et al., 1997; Syrjakoski et al., 2003; Young et al., 2000), although one study observed long repeats only among the MBC cases (Young et al., 2000).
Risk of breast cancer in men is also related to hormonal changes, including estrogen changes due to mutation. The ER protein is a critical component of hormonal regulation of breast tissue, which is underscored by its presence being a positive prognostic indicator for hormone therapy in breast tumors. MBC is associated with diseases or body conditions that increase estrogen exposure or that have effects similar to increased estrogen exposure (Wang-Rodriguez et al., 2002). Progesterone influences differentiation, proliferation, and other functions of the mammary gland by mechanisms that are more complicated and less understood. In one of the findings by Mourao Netto et al., (2001), 102 patients with MBC were diagnosed out of which 48 cases were subjected to immunohistochemical analysis. A total of 36 (75%) and 33 (68.8%) of the 48 cases were positive for ER and PR, respectively. Clearly, more evidence is needed to elucidate the participating role of AR, ER and PR genes in MBC.
Clinical Details
Almost all of the clinical features of breast cancer that have been described in women have also been reported in men. MBC typically presents as a lump or swelling, skin dimpling, nipple retraction and discharge from the nipple (Sandler et al., 1994). However, most breast lumps in men are due to gynecomastia and not cancer, which affects approximately 30% of healthy men (Williams, 1963). A thorough clinical breast examination will be performed to locate the lump or suspicious area and to feel its texture, size, and relationship to the skin and muscle tissue. Accurate diagnosis with mammography can be helpful in studying malignant breast disease (Evans et al., 2001; Gunhan-Bilgen et al., 2002; Newman, 1997). Ultrasound is sometimes used to evaluate breast abnormalities. Nipple aspirate fluid test (NAF) can also be done by examining fluid under a microscope to determine whether cancer cells are present or not. If cancer cells are not seen in the nipple secretions but a suspicious mass is present, a biopsy of the mass is needed. Several types of biopsies such as fine needle aspiration biopsy, core biopsy, and surgical biopsy can also be a useful adjunct and provide information regarding tumor type (Liberman et al., 1998). ER, PR and her 2-neu status testing should be evaluated in every patient, as these may affect the clinical management (Ross and Fletcher, 1998; Bloom et al., 2001). The rate of hormone-receptor positively increases in female breast carcinoma in contrast; the rate of her-2/neu gene is less likely over expressed in male breast carcinoma (Ross et al., 2004). Imaging test such as chest X-ray, bone scan, computed tomography, magnetic resonance imaging is also useful for the extent of disease.
Prognostic factors that have been evaluated include the size of lesion and the presence or absence of lymph node involvement, both of which correlate well with prognosis (Giordano et al., 2004). Men with tumors measuring 2-5 cm have a 40% higher risk of death than men with tumors < 2 cm in maximum diameter (Giordano et al., 2004). Similarly, men with lymph node involvement have a 50% higher risk of death than those without lymph involvement (Giordano et al., 2004). Many new prognostic factors, such as changes of the p53 tumor suppressor gene, the EGFR and microvessel density, are currently being studied.
Treatment
For years the medical profession assumed that MBC was significantly different from FBC. Today they are grouped together and receive the same treatment regimens. The mainstay of breast cancer is surgery, since the MBC treatment is confined to the area directly behind the nipple; treatment for males has always been a radical mastectomy (Weber-Chappuis et al., 1996). Historically, radical mastectomy was often performed but retrospective studies indicate that the outcome for men is equally good when treated with less invasive surgery (Maddox et al., 1983).
Radiation therapy consists of the use of high-powered X-ray or gamma rays that precisely target the area that is being treated, is recommended in men as in women. Perkins et al., (2002) studied a series of 142 male patients treated at the University of Texas M.D Anderson Cancer Center, to determine which male patients would derive benefit from adjuvant radiation therapy. Although radiation therapy can reduce the chance that breast cancer will recur in the breast, it is much less effective in prolonging patient survival.
Systemic treatments include chemotherapy, immune therapy and adjuvant hormonal therapy. Chemotherapy can be given both before and after surgery. One prospective study of adjuvant chemotherapy in men has been published by Patel et al.,(1989), out of 11 male patients treated with adjuvant chemotherapy, 4 patients experienced a relapse, 7 were disease free and 1 died of metastatic breast cancer. There are several different chemotherapy regimens that may be used. Because men have high rates of hormone receptor positivity, adjuvant hormonal therapy is very promising. Patients with estrogen receptor positive tumors will typically receive a hormonal treatment after chemotherapy is completed. Many retrospective studies have evaluated the effectiveness of tamoxifen, raloxifene, transtuzumab in MBC (Ribeiro Swindell, 1992; Anelli et al., 1994). Studies also suggest that chemotherapy combinations containing anthracycline drugs (such as doxorubicin or epirubicin) treat breast cancer with too much HER2/neu more effectively than combination that do not include these drugs. aromatase inhibitors are typically given to postmenopausal women to lower the amount of estrogens in their systems. One case series of five patients with metastatic disease treated with aromatase inhibitors has been published by Giordano et al., (2002). Clearly, further investigation is needed to determine the efficacy of aromatase inhibitors in male patients.
Proteomic Approaches in Breast Cancer Research
With the progress of the human genome project, a large-scale analysis of proteins within a single experiment called proteomics, has gained much attention. In combination with genomics, proteomics can provide a holistic understanding of the biology underlying disease processes. This field incorporates tools and technologies that can be applied to body fluids and tissue in order to extract important biological information to aid clinicians and scientist in understanding the biology of their system of interest, such as patient with cancer. Cancer proteomics encompasses the identification and quantitative analysis of differentially expressed proteins from normal tissue, premalignant and malignant tissue, which can be used as a biomarker for cancer diagnosis. Appropriate biomarkers may be able to define risks and identify the early stages of tumor development, assist in tumor detection and diagnosis, verify stratification of patients for treatment, predict outcomes of the disease, and help in surveillance for disease recurrence. Many molecular markers are available for the better understanding of FBC tumorigenesis and disease progression and possibly to guide treatment (Levenson, 2007). The prognostic factors and expression of molecular markers in male breast carcinomas are similar to those in FBCs (Ciocca et al., 2006). However, few studies are needed to be performed on the male breast counterpart.
Cancer is commonly described as a genetic disease. The DNA is transcribed into RNA then translated into protein. During this a number of alterations and modifications can occur at transcriptional, translational and posttranslational levels (Larsson et al., 2000; Charlwood et al., 2000) a new biologic paradigm proteomics is therefore needed. Some of proteomics tools applied in cancer research include 2-D gel electrophoresis, western blotting, mass spectrometry approaches, laser capture microdissection (LCM), tissue microarray, ELISA. These tools allow for efficient means of identifying new biomarkers for the early detection and diagnosis of MBC (Figure 2)
The National Cancer Institute has created the Early Detection Research Network, which is focusing on discovery and validation of biomarkers for early cancer detection (Verma et al., 2001). Although rapid, high throughput proteomic technologies are currently being developed and applied to breast cancer research; 2-D PAGE continues to be the workhouse of proteomic-based methods. Typically, proteins samples are denatured and separated on the basis of their charge through isoelectric focusing in first dimension and on the basis of mass through SDS-PAGE in second dimension. In one of the study using sera from the breast cancer patients, 2-D gel electrophoresis (2-DE) and western blot analysis was used for discovering a tumor- specific marker RS/DJ-1. Antibody to RS/DJ-1 was used to confirm that it was the protein antigenic in 4 of 30 breast cancer patients (Le Naour et al., 2001; Kobayashi, 2001).
Matrix-assisted laser desorption and ionization with timeof- flight detection mass spectrometry (MALDI-TOF MS) and chip-based technique, Surface-enhanced laser desorption and ionization with time-of-flight mass spectrometry (SELDI-TOF MS) are the two methods currently being employed. The principle behind SELDI is surface-enhanced affinity capture through the use of specific probe surfaces or chips. In breast cancer, SELDI-TOF MS was used to investigate not only serum/plasma, tumor tissues but also NAF (Hu et al., 2005; Mendrinos et al., 2005; Paweletz et al., 2001) as a potential source for diagnostic biomarkers and recently as a potential tool to predict outcome and/or to monitor cancer treatment. Application of SELDI based technologies to serum screening in breast cancer screening is promising. Li et al., (2002) have identified three protein peaks using SELDI-TOF mass spectrometry that discriminate between stage 0-I cancer patients and non-cancer patients as controls. Other recently identified breast cancer biomarkers using SELDI include Hsp 27, 14-3-3 sigma and mammoglobin/lipophilin complex (Rui et al., 2003; Carter et al., 2002).
Tissue microarray technology allows rapid visualization of molecular targets in thousands of tissue specimens at a time either at the DNA, RNA or protein level. Validation of tissue microarray is currently ongoing in breast carcinoma and helps in protein expression profiling. Camp et al., (2000) demonstrate that many proteins retain their antigenicity for more than 60 years. Recently two array-based technologies, cDNA and oligonucleotide arrays, have been applied to gene expression quantification and to relate how the gene expression pattern of one gene correlates to the expression of other genes in or between different tumor samples. Hedenfalk et al., (2001) studied breast cancer from BRCA1 and BRCA2 carriers using cDNA microarray. They then validated and analyzed protein products encoded by these genes by immunohistochemistry on the tissue array. cDNA microarrays were used to identify patterns of gene expression in human mammary epithelial cells growing in cultures and in primary human breast tumors. In an analysis of breast carcinomas, Perou et al., (1999) identified clusters of proliferation-related genes and interferon-regulated genes exhibiting differential expression. This technique will be extremely useful in identifying other proteins involved in breast cancer.
LCM technology has made it possible to carry out proteomics studies with pure cell proliferation harvested directly from frozen tumor sections. LCM does not disrupt the histological architecture of the tissue, and it preserves the state of the in vivo cellular molecules. The application of such technology becomes particularly important in the proteomic comparison of normal and malignant breast tissue. Sgroi et al., (1999) have provided the first microarray/ immunohistochemical data on breast tumors using LCM for epithelial cell purification.
Protein identification has been achieved by peptide mass fingerprinting in which the masses of proteolytic peptide fragments is measured and compared to the corresponding peptide mass fingerprints with published protein sequence databases. Commonly used protein sequence databases include the SWISSPORT, OWL and NCBInr databases. Several software programs for both quantitative and qualitative protein identification are also available on line (WU et al., 2002). These software applications help in identification of the experimental proteins and facilitate the analysis of posttranslational modifications and three-dimensional structure of identified proteins. These proteomic approaches can be further used to identify a potential biomarker for early detection and diagnosis of MBC.
Breast cancer commonly occurs in women, but now the incidence is also seen in men. Risk factors include age, family history, genes, liver diseases (cirrhosis), alcohol, diet, and obesity. Klinefelter syndrome, in which patients carry XXY chromosome, may be present in men with breast cancer for this reason they often develop gynecomastia. Similar to FBC, genetics seems to play an important role in MBC. Both BRCA1 and BRCA2 genes are associated with most hereditary male breast carcinoma, but only BRCA2 mutations confer a significant risk to men. Other genes that is responsible for male breast carcinoma includes c-erbB-2 gene, p53 gene, bcl-2, PTEN (cowden’s syndrome), a CHEK2, CYP17, AR, ER and PR gene. A more accurate assessment of breast cancer genes will provide us with an enhanced chance to win the battle against male breast carcinoma.
The diagnostic studies revealed that mammography could be helpful in studying malignant breast disease. Ultrasound is sometimes used to evaluate breast abnormalities. NAF can also be done by examining fluid under a microscope to determine whether cancer cells are present or not. ER, PR and her 2-neu status testing concludes that tumors of the male breast are more likely express the estrogen and progesterone receptors and less likely overexpresses her2- neu gene than breast cancers in women. Radical mastectomy was often performed for treatment of MBC but retrospective studies indicate that the outcome for men is equally good when treated with less invasive surgery. Radiation therapy is also recommended in men as in women but it is much less effective in prolonging patient survival. Metastatic diseases can be treated with either chemotherapy or adjuvant hormonal therapy. Although, tamoxifen is the most accepted front-line of the adjuvant hormonal therapies; the studies on efficacy of aromatase inhibitors in male patients is still needed.
Early detection is critical in breast cancer control and prevention but proteomics approaches helps by providing valuable information about the protein expression of breast cancer patients. (2-DE) and western blot analysis was used for discovering a tumor- specific marker RS/DJ-1. Application of SELDI- based technologies to serum screening in breast cancer screening is promising.
Breast cancer biomarkers using SELDI were identified including Hsp 27, 14-3-3 sigma and mammoglobin/ lipophilin complex. Using cDNA microarrays clusters of proliferation-related genes and interferon-regulated genes exhibiting differential expression were identified. Tissue microarrays will be developed for profiling protein expression of MBC in a matter of few days. LCM has helped in sampling specific cell populations directly from tissue samples without causing any mechanical disruption to cell.
Given the promise of proteomics technologies, there are limitations that need to be overcome. Highly abundant proteins often hinder the identification of low-abundance proteins. To overcome this problem enrichment and prefractionation strategies such as affinity-based columns should be used to remove the high abundant proteins and the use of narrow pH range gels will increase better resolution of protein molecules. Therefore it can be anticipated that this, along with the application of vital information on susceptible genes will greatly ameliorate the ability to find tumor markers for early detection and treatment of male breast carcinoma.