Review Article - (2023)Volume 9, Issue 1
Prophylactic and Therapeutic EBV Vaccines of rBCG
Qiu Ling Wang1*, Qing-Jie Xue2 and Li Zhang3Abstract
Based on the research of recombinant BCG (rBCG) vector vaccine for many years, we recombined BZLF1 and LMP2 gene into BCG vector and studied its immunological mechanism and function in vivo and in vitro, which laid a solid foundation for the application of recombinant BCG vector vaccine, in this paper, the reason of using BZLF1 and LMP2 gene and the function of BCG vector are summarized.
Keywords
BCG; BZLF1; LMP2; Vaccine
Introduction
On the basis of previous studies, we transformed the BZLF1 and LMP2 fusion genes of EBV specific antigen into BCG vector and expressed them efficiently in BCG to obtain rBCG with stable secretory expression, then we studied the effect of rBCG secreted Epstein-Barr Virus (EBV) fusion protein on the proliferation of tumor cells and the antitumor activity of effector cells in vitro, and established the tumor-bearing mouse model, studied the different preventive and therapeutic effects of rBCG on EBV positive tumors and its immune mechanism, a rBCG vaccine that can effectively prevent or treat EBV positive tumors was gotted, in order to prevent and treat EB virus infection and tumor.
EBV is a 184 kb lymphocyte Herpesvirus that was discovered in 1964 by Epstein and Barr in the study of Malignant Lymphoma in African children. EBV latent infection has been found to be associated with a variety of human cancers, including Nasopharyngeal Carcinoma, Burkitts Lymphoma, Hodgkins Lymphoma, and Gastric Cancer, so in 1997 the International Agency for Research on Cancer (IARC) classified EBV as a firstclass carcinogen. Since the discovery of EBV, great progress has been made in the study of EBV-related vaccines. However, because of the complexity of EBV life cycle, there is no mature vaccine on the market. It is urgent to develop a safe and effective vaccine to prevent many diseases and tumors caused by EBV. The potential carcinogenicity of EBV, live attenuated vaccine of EBV is not suitable for prevention in healthy people. The research of EBV vaccine based on EBV subunit vaccine has become a hot spot. But there is no literature and patent report around the world and no report of clinical application also. With the development of molecular biology and tumor immunology, people pay more and more attention to using immunotherapy to prevent the recurrence and distant metastasis of NPC.
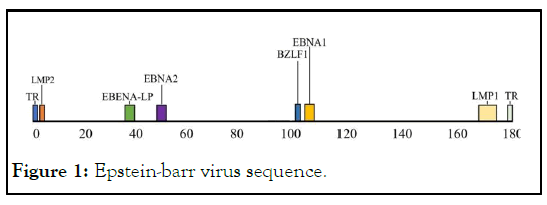
Figure 1: Epstein-barr virus sequence.
Literature Review
LMP2 including LMP2A and LMP2B, only the first exon of Nterminal is different from each other. LMP2 has been proved to induce stronger specific cellular immune response, and many CTL epitopes have been identified [1]. In recent years, the expression of LMP2 gene in all B cells of EBV latent infection in vivo and in vitro has been demonstrated, moreover, LMP2 is one of the few conservative antigens that are stably expressed in nasopharyngeal carcinoma, lymphoma and other tumor cells. It has potential T cell activation epitope and can mediate the function of killer T cells. Therefore, LMP2 may be an ideal target antigen for EBV related tumor immunotherapy. If the gene is transferred into the expression vector alone or in combination, it may express this target antigen and induce specific cellular and humoral immunity, prevention of EBV infection and killing of EBV positive tumor cells [2,3].
In this study, we used another key Epstein Barr virus gene, the immediate early gene (BZLF1 ), the immediate early gene expression can induce the virus from latent period to lytic period, the encoded protein is a transcription activator. EBV infects host cells including latent period and lytic period. In EBV positive tumor cells, EBV is usually in latent period, and its latent existence and DNA integration with host cells are the important reasons leading to the occurrence of tumor, artificial induction of EBV from latent phase to lytic phase, leading to the death of tumor cells, is expected to be a new method to treat EBV-associated tumors. BZLF1 of EBV encodes a transcriptional activator, and the expression of BZLF1 can induce EBV to enter the viral replication cycle from incubation period. It can induce latent EBV in tumor cells to enter the lytic phase, and open the mechanism of virus replication, EBV proliferation leads to the lytic and death of EBV positive tumor cells, which can specifically kill tumor cells. In this study, we propose to fuse BZLF1 coding protein expression. The expression of BZLF1 coding protein immediately causes the latent EBV to enter the lytic phase, the immunogenicity of the protein encoded by the early gene BZLF1 can also induce specific cellular and humoral immunity, which has been confirmed in our previous studies.
We linked the two genes BZLF1 and LMP2 together by the DNA sequence of the peptide junction (Gly4Ser)3, which was composed of 15 amino acids. By constructing the fusion gene, LMP2 induced specific CTL and BZLF1 -induced latent EBV replication into the lytic stage, thus killing EBV positive tumor cells. How to make the fusion gene play an effective and safe anti-tumor role in nude mice model of nasopharyngeal carcinoma? It provides an objective basis for further clinical research and treatment of patients with nasopharyngeal carcinoma.
Discussion
The molecular biology modification of BCG can make its therapeutic effect on tumor more obvious. Therefore, if BCG is used as the expression vector of these cytokine genes, the effect may be better if it is combined with adjuvant and vector. This genetic engineering vaccine may play the following roles: BCG and cytokines which it stimulates the secretion and expression of the body can activate and promote the proliferation of immune cells and exert their function of killing tumor cells, increase the activity of killing tumor cells. The cytokines secreted by BCG after direct intratumoral injection directly affect the immunocytes and tumor cells, which can avoid the side effects of systemic administration of cytokines according to the specificity of the treated tumor. The antigen protein produced by the rBCG can induce tumor-specific CTL.
The molecular modifications of BCG maybe make its therapeutic effect on tumor more obvious. Therefore, if BCG is used as the expression vector of some important proteins, it may be more ideal to combine adjuvant and vector together. These kind of genetic engineering vaccines may play the following role: BCG and the cytokines can activate and promote the proliferation of immune cells, and these would play the role of killing tumor cells, improve the activity of killing tumor cells. The proteins secreted by BCG after direct intratumoral injection directly affect tumor immune cells and tumor cells, it can completely avoid the side effects of systemic use with cytokines on the whole body. The relevant surface antigen protein produced by rBCG can be introduced into the tumor according to the specificity of the tumor to induce the production of tumor-specific CTL. The rBCG can enter into the tumor cells and persist in these cells, then the tumor-specific CTL can be induced by the secreted antigen protein, with tumor cells proliferate, they divide and pass on.
The aim gene was transferred into BCG. We used the pMV261 secretory expression plasmid which was constructed by Stover et al. This plasmid can make exogenous gene express in BCG secretively and stably, which will promote the research of rBCG greatly.
The plasmid pMV-BFP2 was transformed into BCG vaccine by electroporation to obtain rBCG which is resistant to EBV virus and can eliminate EBV positive tumor cells, to produce an immune response superior to that of a single gene? In vitro and in vivo anti-tumor experiments were used to examine the effects of target gene expression on tumor cell apoptosis and proliferation, and the inhibition of tumor growth in tumorbearing mice, the effect of fusion protein on apoptosis of tumor cells was analyzed at cellular and molecular levels to prepare subunit vaccine with high immunity to EBV positive tumor cells [4-7].
Conclusion
In the future, the other EBV specific gene fragments EBNA1, LMP1 will be fused with BZLF1 respectively, and the fused gene will be transferred into BCG expression vector, to study the antitumor effect of rBCG expressing EBV fusion protein in vitro and the anti-tumor activity of effector cells, then we will study the different therapeutic effects of rBCG on EBV positive tumors and their immune mechanisms, to evaluate their anti-tumor effects and safety, in aim to construct BCG vector vaccine that can effectively treat EBV positive tumors, in order to prevent and treat EBV infection and tumor, and benefit EBV positive tumor patients, especially nasopharyngeal carcinoma patients, lay the theoretical and experimental foundation. In vitro and in vivo anti-tumor experiments were carried out to investigate the effect of target gene expression on apoptosis and proliferation of tumor cells, the effect of the fusion protein on the apoptosis of tumor cells was analyzed at the cellular and molecular levels. Then a subunit vaccine was prepared which could produce high immunity to EB virus positive tumor cells. If the experiment can achieve the expected results, it will bring great economic and social benefits; it will lay a theoretical and experimental foundation for further clinical research and treatment of EBV positive tumor patients, ideally, volunteers will be recruited for the pre-clinical phase of the trial.
References
- Kraus RJ, Perrigoue JG, Mertz JEZ. ZEB negatively regulates the lytic-switch BZLF1 gene promoter of Epstein-Barr virus. J Virol. 2003;77:199-207.
- Xue QJ, Li YQ, Yang CQ, Chen T, Li XZ, Cheng B, et al. Anti-tumour research of recombinant BCG using BZLF1 and hGM-CSF fusion genes. Vaccine. 2017;35(12):1599-1607.
- Rosevear HM, Lightfoot AJ, O’Donnell MA. The role of neutrophils and TNF-related apoptosis-inducing ligand (TRAIL) in Bacillus Calmette-Guerin (BCG) immunotherapy for urothelial carcinoma of the bladder. Cancer Metastasis Rev. 2009;28(4):345-353.
- Smith C, Khanna R. The development of prophylactic and therapeutic EBV vaccines. Curr Top Microbiol Immunol. 2015;391:455-473.
- Takuma H, Horiuchi A, Sano K, Hiraoka N, Kanai Y, Shiozawa T, et al. Mice-lacking LMP2, immuno-proteasome subunit, as an animal model of spontaneous uterine leiomyosarcoma. Protein Cell. 2010;1(8):711–717.
- Luo Y, Yamada H, Chen X, Ryan AA, Evanoff DP, Triccas JA, et al. Recombinant mycobacterium Bovisbacillus Calmette-Guerin (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin Exp Immunol. 2006;137(1):24-34.
- Wang JJ, Qie Y, Zhu B, Zhang H, Xu Y, Wang Q, et al. Evaluation of a recombinant BCG expressing antigen Ag85B and PPE protein Rv3425 from DNA segment RD11 of Mycobacterium tubercμlosis in C57BL/6 mice. Med Microbiol Immunol. 2009;198(1):5-11.
Author Info
Qiu Ling Wang1*, Qing-Jie Xue2 and Li Zhang32Department of Medicine, Jining Medical University, Jining, China
3Department of Endocrinology, Yantai Yuhuangding Hospital of Qingdao University, Shandong, China
Citation: Wang QL, Xue QJ, Zhang L (2022) Prophylactic and Therapeutic EBV Vaccines of rBCG. Appli Microbiol Open Access. 8:242.
Received: 08-Jul-2022, Manuscript No. AMOA-22-18259; Editor assigned: 11-Jul-2022, Pre QC No. AMOA-22-18259(PQ); Reviewed: 25-Jul-2022, QC No. AMOA-22-18259; Revised: 26-Jan-2023, Manuscript No. AMOA-22-18259(R); Published: 06-Feb-2023 , DOI: 10.35248/2471-9315.22.8.242
Copyright: © 2022 Wang QL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.