Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2022)Volume 10, Issue 6
Purpose: The importance of Measurable Residual Disease (MRD) detection in B-acute lymphoblastic leukemias (BALL), has led to identifying ways to increase the sensitivity of detecting MRD by multicolor flow cytometry (MFC) and distinguishing it from the hematogones. The identification of cost-effective panel by MFC is necessary and hence this study was undertaken with an objective to propose a presumptive single tube ten-color antibodies panel for MRD detection by the analysis of immunophenotypic profile of BALL with respect to seven newer Leukemia-Associated Immunophenotype (LAIP) markers at diagnosis namely CD9, CD44, CD58, CD73, CD81, CD86 and CD123, in addition to six backbone markers (CD45, CD34, CD38, CD10, CD19 and CD20) which would be useful in a resource constrained setting.
Methods: This was a retrospective, cross-sectional study. All newly diagnosed cases of BALL which were diagnosed based on morphology, cytochemistry and immunophenotyping by MFC from the period of October 2019 to April 2021 were included (n=82). The expression pattern of the markers at diagnosis were studied in comparison to that of normal pattern of hematogones maturation.
Results: Over-expression of CD73 (83%) and CD86 (77%) were the most effective markers in establishing LAIP at diagnosis. Under-expression of CD81 was the next most frequent LAIP in 71% cases. CD44 and CD58 were similar to each other in terms of LAIP expression and hence any one of them could be used. CD9 though positive in 94% of the cases showed significant overlap with the marker expression of hematogones (overexpression in only 37%) and CD123 was overexpressed in only 36%.
Conclusions: We propose a single ten-color tube comprising of the markers CD45, CD19, CD34, CD10, CD20, CD38, CD73, CD86, CD81 and CD44 for diagnosis as well as for MRD analysis in the post-therapy samples of BALL in low- and middle-income countries.
Measurable residual disease; B-acute lymphoblastic leukemia; Leukemia associated immunophenotype; Multicolor flow cytometry
Measurable Residual Disease (MRD) is defined as those leukemic population of cells which are undetectable by morphology but detectable by immunophenotypic, molecular, or cytogenetic assessment in the bone marrow [1]. MRD detection is very important for assessing the prognosis and is an early marker for therapeutic response assessment in both childhood and adult B-Acute Lymphoblastic Leukemia (BALL) [2]. Subsequently, it helps in either escalating or de-escalating the therapy and the use of the best possible treatment options [1-3]. The two well established techniques for identifying MRD are Multicolour Flow Cytometry (MFC) and PCR based molecular technique [4]. MFC provides real time assessment and is relatively quicker, widely available and user friendly. This is in contrast to molecular techniques which are time consuming, require higher degree of expertise and also target a specific molecule which should be available in each case [4]. This has made MFC popular and with increasing number of fluorochromes and antibodies available, its sensitivity and specificity are gradually approaching that of molecular techniques [4-7].
The greatest challenge in MRD detection by MFC lies in identifying the residual leukemic blasts amidst a background rich in normal B-cell precursors/hematogones [6,7]. Hematogones are increased in a regenerating marrow following therapy, morphologically mimic blasts and immunophenotypically also express CD45, CD34, CD38, nTdT, CD10, CD19, and CD20 which overlaps with blasts [6]. The correct delineation of hematogones from residual blasts requires a wide knowledge about the expression pattern of markers in different stages of hematogones. There are two commonly used approaches for MRD detection by MFC namely “Deviation from Normal” (DFN) method and “Leukemia Associated Immunophenotype” (LAIP) both of which are closely interrelated [8]. In order to identify a small population of residual leukemic blasts, the widely accepted criterion is to identify a minimum of two LAIPs [8]. LAIP is defined at diagnosis and then used to track the presence of MRD during treatment [8,9]. Applicability of the commonly used backbone markers in MFC is limited in some cases by the lack of demonstrable LAIP [10]. Another well documented issue is immunomodulation and loss of the diagnostic immunophenotypic signature following therapy [11,12]. Hence, identification of those new markers that are differentially expressed by the leukemic blasts, are easily detectable at diagnosis and relatively stable following therapy is warranted.
The addition of several new markers to the set of backbone markers (which includes CD45, CD34, CD38, CD10, CD19, and CD20) increases the number of tubes required for the diagnostic and MRD panels. This imposes cost constraints and decreases the sensitivity of MRD analysis because each additional tube contains fewer cells. Hence, selecting the most efficient combination of the newer markers is critical for routine use in resource-constrained settings. With this in mind, the present study's objective was to propose a presumptive single-tube, ten-color antibody panel for MRD detection by MFC. This was accomplished by evaluating the immunophenotypic profiles of BALL cases with respect to seven newer LAIP markers-namely, CD9, CD44, CD58, CD73, CD81, CD86, and CD123-in combination with six backbone markers (CD45, CD34, CD38, CD10, CD19, and CD20).
This was a retrospective and cross-sectional study. All newly diagnosed cases of BALL from the period of October 2019 to April 2021 were included in the study (n=82). The diagnosis was made based on morphology, cytochemistry and immunophenotyping by MFC. For majority of the cases, at the time of diagnosis, bone marrow samples received in EDTA were processed within 2-4 hours of receipt of the sample. In some cases, peripheral blood sample was used for MFC at the time of diagnosis.
Antibody panels used
Sequential staining approach with acute leukemia orientation tube having relevant lineage and immaturity markers was run followed by the extended B-cell leukemia panel. The pre-defined antibody panels which was adapted and modified from Euro Flow Consortium recommendation with incorporation of new LAIP markers were used [13,14]. The following three tubes were used (Table 1), labelled as B1, B2 and B3 which comprised of the pre-titrated volume of backbone markers (CD45, CD34, CD10, CD38, CD19 and CD20) along with seven newer markers (CD9, CD44, CD58, CD73, CD81, CD86 and CD123) in two tubes (B1 and B2). The third tube (B3) comprised of CD45, CD19, CRLF2, CD13, CD33, CD15, CD11b and CD11c which was used to assess aberrant expression of these markers.
| Fluorochrome | FITC | PE | ECD | PC5.5 | PC7 | APC | APC 700 | APC 750 | PB | KO |
|---|---|---|---|---|---|---|---|---|---|---|
| ALOT* tube | nTdT | cMPO | CD19 | cCD79a | CD34 | cCD3 | CD45 | CD7 | sCD3 | HLA-DR |
| HT-1, 4, 8, 9 | CLB-MPO-1 | J3-119 | HM47 | 581 | UCHT-1 | J33 | 8H8.1 | UCHT-1 | IMMU-357 | |
| B1 tube | CD81 | X | CD123 | CD19 | CD34 | CD10 | CD20 | CD38 | CD9 | CD45 |
| JS64 | - | SSDCLV 107D2 | J3-119 | 581 | ALB1 | HRC20 | LS198-4-3 | ALB6 | J33 | |
| B2 tube | CD58 | CD73 AD-2 | CD19 | CD86 | CD34 | CD10 | CD20 | CD38 | CD44 | CD45 |
| A1CD 58 | - | J3-119 | HA5.2B7 | 581 | ALB1 | HRC20 | LS198-4-3 | J.173 | J33 | |
| B3 tube | CD13 | CRLF | CD19 | CD33 | CD34 | CD11c | CD45 | X | CD11b | CD15 |
| SJ1D1 | 1F11/ TSLPR | J3-119 | D3HL60.251 | 581 | BU15 | J33 | - | Bear1 | 80H5 |
Note: *ALOT (Acute Leukemia Orientation Tube) and B3 tube were used in all cases only at the time of diagnosis. B1 and B2 tubes were used to identity LAIP at diagnosis and to track MRD on follow-up. B3 tube was used for MRD only in selective cases with LAIP.
Table1: Panel of antibodies, their fluorochrome and clone used.
Sample processing
All the reagents and consumables were procured from Beckman Coulter India private limited Company. Stain-lyse-wash method was used for sample processing at the time of diagnosis. Titration experiments were carried out and the concentration at which better signal to noise ratio were identified and the antibody concentration of 2.5-5 microliter was used for majority of the antibodies. Total leucocyte count was calculated from hematology analyzer and the sample was diluted appropriately if it was >50,000 cells/cu.mm.
Staining is done with the fluorochromes tagged surface or intracellular antibodies. Initially the staining with pre-titrated volume of surface antibodies was performed with 100 microliter of the samples for majority of the cases. This step was followed by vortexing and incubating the mixture in the dark for 20 minutes. The washing of excess of unbound antibodies was performed by adding 2 ml of phosphate buffered saline, vortexing for few seconds, centrifuging for five minutes at 2,500 RPM and discarding the supernatant. Formaldehyde was used for fixation and saponin for permeabilization step for the intracytoplasmic or nuclear antibodies (Intraprep R1 and R2™). This step was followed by adding intracytoplasmic or nuclear antibodies, incubation and washing step. In case of only surface antibodies, Optilyse™ was used to lyse the red cells after the staining step and followed by washing procedure. Unstained tube was processed along with each panel to check for auto fluorescence separately for surface and intracellular antibodies. The diluent (Iso sheath fluid™) was used for suspending the cells and used for acquisition. A solution of proteolytic enzyme (Cleanse™) was used to remove the unwanted debris in the instrument at the end of the procedure.
Acquisition and analysis
Samples were acquired on a three-laser and ten-color flow cytometer comprised of two fluorescent channels from the violet (405 nm) laser, five channels from the blue (488 nm) laser and three channels from the red (638 nm) laser (Navios, Beckman Coulter TM). The optical alignment, electronic standardization, sensitivity/linearity of the flow cytometer was calibrated at the time of installation with re-calibration done every year. Flow cytometer performance was monitored daily using commercially available fluorescent beads (Flow check proTM). Photomultiplier tube voltages and compensation were standardized by cell- based methods and by using beads, plotted on Levy Jennings chart and verified on day to day basis. For the diagnostic immunophenotyping, at least 1,00,000 events per tube were acquired.
Data was interpreted using Kaluza software (version 2.1). Initial gating was done with side scatter versus CD45 gating strategy to identify blasts; lymphocytes and granulocytes were used as normal controls. For all the diagnostic samples, antigen expression of blast was reported according to AIEOP-BFM consensus guidelines and WHO style tripartite consensus reporting as negative, weak and strong based on Median Fluorescent Intensity (MFI) [15]. When the entire blast population overlapped with negative control or positive blast subset was <10%, it was labelled as negative; when majority of the blast overlapped with the negative control and/ or positive blast subset was between 10%-50%, it was labelled as weak positive and when only a minority of blast overlapped with negative control and positive blast subset was >50% it was labelled as strong positive [15].
Further, the expression pattern of the markers was also studied in comparison to that of normal pattern of maturation of hematogones by using a template. This template was prepared by running ten uninvolved staging bone marrow samples (non- Hodgkin lymphoma and solid organ malignancy) of pediatric as well as adult patients as normal control. This template was generated using the dot plots with multiple combinations of markers used in the panel (Figure 1).
LAIP was identified based on the abnormal expression of individual markers with respect to hematogones as, ‘over- expression’ if they were strongly expressed or had a MFI value more than hematogones and the markers with negative to weak expression or MFI value less than hematogones was labelled as ‘under-expression’. For practical purposes and to routinely interpret the over-expression and under-expression of a marker, a minimum of half log difference between negative and positive expression (between normal and abnormal) was considered significant. Marker combinations which are not co-expressed by normal B-cells were referred to as asynchronous expression (CD34 and CD20). Cross lineage expression of myeloid antigens such as CD13, CD33, CD11c and CD15 were also noted.
Of the 82 cases, 47 (57%) were males and 35 (43%) were females. The age ranged from six months to 70 years with mean age being 19.16 years, 43% of cases being in the first decade of life. Total WBC count at diagnosis ranged from 0.11 × 109/L to 733.4 × 109/L with a mean WBC count of 48.08 × 109/L. Nearly 56% cases presented with leukocytosis, 26% and 18% had leucopenia and normal WBC count at presentation respectively. Around one-fourth (26%) of the cases presented with pancytopenia and 15% of these cases had sub-leukemic picture (less than 20% blasts in the peripheral blood but bone marrow confirms the diagnosis of acute leukemia).
Normal expression of the backbone markers and the seven newer markers were studied on hematogones stage I and II in control bone marrow samples (Figure 1). The expression patterns of the blast were compared with the hematogones at the time of diagnosis (Figure 2). Table 2 shows the frequency of individual marker positivity at diagnosis.
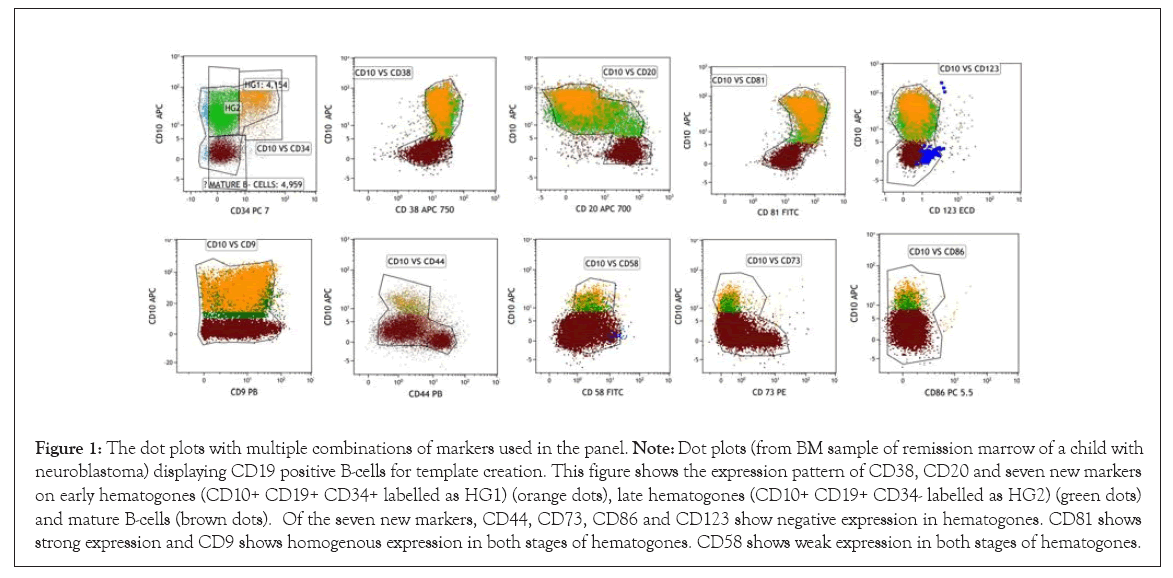
Figure 1: The dot plots with multiple combinations of markers used in the panel. Note: Dot plots (from BM sample of remission marrow of a child with neuroblastoma) displaying CD19 positive B-cells for template creation. This figure shows the expression pattern of CD38, CD20 and seven new markers on early hematogones (CD10+ CD19+ CD34+ labelled as HG1) (orange dots), late hematogones (CD10+ CD19+ CD34- labelled as HG2) (green dots) and mature B-cells (brown dots). Of the seven new markers, CD44, CD73, CD86 and CD123 show negative expression in hematogones. CD81 shows strong expression and CD9 shows homogenous expression in both stages of hematogones. CD58 shows weak expression in both stages of hematogones.
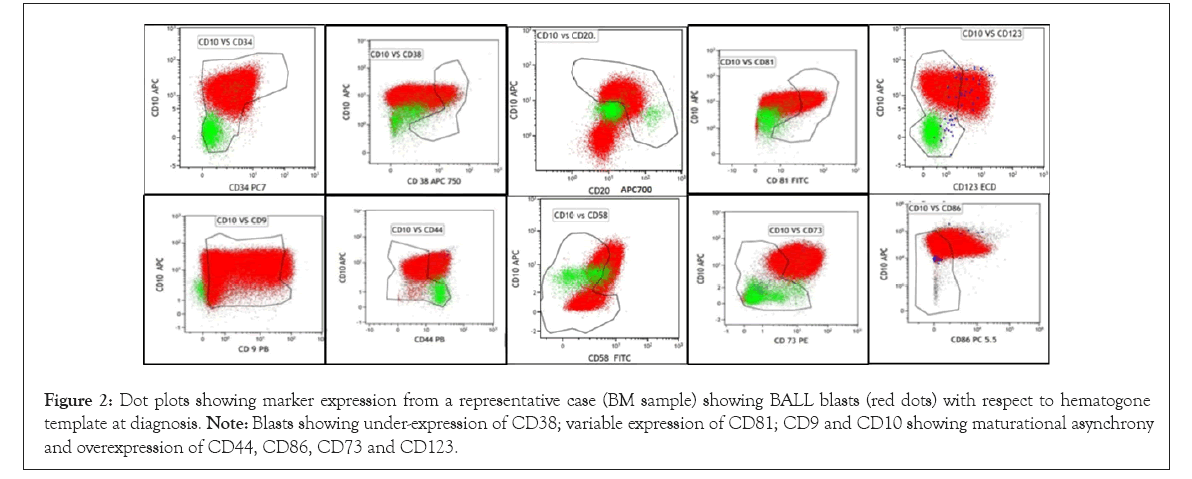
Figure 2: Dot plots showing marker expression from a representative case (BM sample) showing BALL blasts (red dots) with respect to hematogone template at diagnosis. Note: Blasts showing under-expression of CD38; variable expression of CD81; CD9 and CD10 showing maturational asynchrony and overexpression of CD44, CD86, CD73 and CD123.
| Commonly used markers | Number of cases analysed | Frequency of positivity (weak and strong) |
|---|---|---|
| CD19 | 82 | 82/82 (100%) |
| CD10 | 82 | 77/82 (93.90%) |
| CD34 | 82 | 65/82 (79.27%) |
| CD38 | 80 | 77/80 (96.25%) |
| CD20 | 82 | 60/82 (73.17%) |
| nTdT | 69 | 65/69 (94.20%) |
| CD22 | 23 | 20/23 (86.95%) |
| cCD79a | 82 | 80/82 (97.56%) |
| Newer markers | ||
| CD9 | 70 | 66/70 (94.28%) |
| CD81 | 82 | 81/82 (98.78%) |
| CD86 | 65 | 54/65 (83.07%) |
| CD44 | 75 | 56/75 (74.67%) |
| CD58 | 82 | 59/82 (71.95%) |
| CD73 | 82 | 67/82 (81.70%) |
| CD123 | 58 | 28/58 (48.27%) |
| Aberrant markers | ||
| CD13 | 82 | 18/82 (21.95%) |
| CD33 | 82 | 6/82 (7.31%) |
| CD15 | 82 | 3/82 (3.65%) |
| CD11c | 82 | 4/82 (4.87%) |
Table 2: Immunophenotyping of BCP-ALL cases using common, newer and aberrant markers at diagnosis and their frequency of positivity.
In order to identify the small population of residual leukemic blasts, the widely accepted criterion is to identify a minimum of two LAIPs. With the present panel, all the cases showed presence of more than two LAIPs. Table 3 highlights the frequency of LAIP expression by backbone and newer markers.
| Backbone markers | LAIP in comparison to hematogones | Frequency of LAIP |
|---|---|---|
| CD10 and CD20 | Asynchronous expression | 31/82 (37.80%) |
| CD 34 and CD20 | Asynchronous expression | 24/82 (29.26%) |
| CD10 and CD9 | Asynchronous expression | 26/70 (37.14%) |
| CD10 | Overexpression | 20/82 (24.39%) |
| Negative/under-expression | 18/82 (21.95%) | |
| CD34 | Overexpression | 15/82 (18.29%) |
| Negative/under-expression | 20/82 (24.39%) | |
| CD20 | Overexpression | 20/82 (24.39%) |
| CD38 | Under-expression | 48/80 (60.00%) |
| CD45 | Under-expression | 52/82 (63.41%) |
| Newer markers | ||
| CD9 | Over-expression | 26/70 (37.14%) |
| CD81 | Under-expression | 58/82 (70.73%) |
| CD86 | Over-expression | 50/65 (76.92%) |
| CD44 | Over-expression | 39/75 (52.00%) |
| CD58 | Over-expression | 32/82 (39.02%) |
| CD73 | Over-expression | 68/82 (82.92%) |
| CD123 | Over-expression | 21/58 (36.20%) |
Table 3: Frequency of LAIP expression by the backbone and newer markers.
We studied a virtual combination of the markers to analyse the ten best marker combinations in terms of highest LAIP expression (Table 4). This was done by analysing the number of LAIPs that would be obtained by using the markers in different permutations and combinations in a single tube. This was done in attempt to merge the currently used B1 and B2 tubes into a single tube.
| Backbone markers | New markers | 0LAIP | 1LAIP | 2LAIP | >2LAIP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | X | X | X | X | 0 | 17.07% | 40.24% | 42.68% |
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | CD73 | CD86 | CD81 | CD44 | 0 | 0 | 1.50% | 98.50% |
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | CD73 | CD86 | CD81 | CD58 | 0 | 0 | 3.03% | 96.97% |
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | CD73 | CD86 | CD81 | CD9 | 0 | 0 | 3.03% | 96.97% |
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | CD73 | CD86 | CD123 | CD58 | 0 | 0 | 6.97% | 93.02% |
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | CD73 | CD86 | CD81 | CD123 | 0 | 0 | 7.14% | 92.80% |
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | CD81 | CD44 | CD58 | CD9 | 0 | 1.47% | 7.35% | 91.17% |
| CD19 | CD20 | CD45 | CD38 | CD34 | CD10 | CD44 | CD58 | CD9 | CD123 | 0 | 0 | 15.90% | 84.1 |
| CD19 | CD20 | CD45 | X | CD34 | CD10 | CD73 | CD86 | CD81 | CD44 | 0 | 1.50% | 3.03% | 95.40% |
| CD19 | CD20 | X | X | CD34 | CD10 | CD73 | CD86 | CD81 | CD44 | 0 | 1.50% | 12.21% | 86.36% |
Table 4: Various virtual ten colour antibody panel combinations which would have maximum chances of correctly identifying MRD on follow-up.
If a combination of the backbone markers alone were used to detect LAIP, 14/82 (17%) cases and 33/82 (40%) cases showed less than two LAIPs and two LAIPs respectively. Only 35/82 (42%) cases showed presence of more than two LAIPs meaning that it would be difficult to track the MRD with just the use of backbone markers; hence not always helped in precise discrimination from the hematogones and emphasizes on the need of new markers. Among the backbone markers, under-expression of CD38 and CD45 were the most frequently expressed LAIP and if we remove these two backbone markers from the proposed single tube MRD panel, the number of cases with more than 2 LAIPs were reduced to 86%.
Of the newer markers, four markers with the highest frequency of LAIP were CD73 (83%), CD86 (77%), CD81 (71%) and CD44 (52%) followed by CD58, CD9 and CD123 (Table 3). Although CD9 showed positivity in 94% of the cases, it had significant overlap with the expression of hematogones and hence was not a very useful marker in terms of differentiation from residual blasts. With respect to CD123, overexpression was noted in 36% of cases.
Instead of all the newer markers in the primary panel, if the four markers with the highest frequency of LAIP being CD73, CD86, CD81 and CD44 were used along with the backbone markers, only one case showed two LAIPS and all the other cases still showed >2 LAIPs (98% cases). However, on just removing the most frequently expressed markers such as CD73 and CD86, the percentage of cases with >2 LAIPs reduced to 91% and further on removing CD81 the percentage further reduced to 84% showing that CD73, CD86 and CD81 were the three most useful markers in the panel and their addition in the panel will make it less vulnerable to missing MRD.
With the above findings, we propose a presumptive single ten-color tube composed of CD45, CD34, CD38, CD10, CD19, CD20, CD73, CD86, CD81 and CD44 for diagnosis as well as for tracking MRD in the post therapy samples. The cost-benefit analysis was performed in order to optimize the resources. The company recommended antibody concentration for most of the antibody is 20 microliter. If we use the same concentration, the cost incurred for the diagnosis with acute leukemia orientation tube, B1, B2 and B3 tube was Rs.15,500/- (INR) (USD 198). Instead if we use the titrated quantity with the theoretically proposed panel, the cost is greatly reduced to Rs. 3,800/- (USD 48).
There have been constant efforts in identifying the best possible ways to increase the sensitivity of detection by MFC and distinguishing from hematogones [10]. Although markers such as CD34, CD10, CD19 and CD20 are routinely used to identify blasts, it is important to remember that immunomodulation, such as up-regulation of CD19 and CD20, down-regulation of CD10 and CD34 occurs following induction therapy [11,12]. Therefore, there is a need to add newer LAIP markers to clearly distinguish regenerating cells from residual leukemic cells.
In India, there are only limited published studies in the field of MRD and the experience with newer LAIP markers has been studied and documented by a few groups [16-20]. Patkar, et al. published the first data from India on standardization of flow-based MRD for BALL with the basic markers, however did not venture much into the use of the newer LAIP markers [16].
Thembare, et al. have studied the pattern of expression of six newer markers such as CD24, CD44, CD72, CD73, CD86, and CD200 in leukemic-blasts in ninety childhood BALL patients and also in hematogones from 30 staging and post-induction marrows of non-BALL BM samples using an eight-color MFC [17]. They studied the differential expression of the newer markers in hematogones and established the expression pattern in them and blasts. In their study, CD73 and CD86 were the most relevant and stable markers in MRD. CD73 showed maximum (83%) frequency of LAIP and CD86 showed highest (100%) stability post therapy [17].
Subsequently, the same group had comprehensively studied a larger cohort of 622 patients of childhood BALL using a ten color MRD assay where they have reported a sensitivity of 2 in 106 in detecting MRD. They emphasized the utility of CD73, CD86, CD123 and CD38 with the highest frequency of LAIPs and with the least post therapy loss of LAIP [18]. Jain, et al. also stressed on the importance of CD73 and CD86 followed by CD123 being the most useful markers. They observed CD73 overexpression in 54.5%. CD86 overexpression in 46.7% and CD 123 overexpression in 50.7% cases [19].
In the present study, we used widely available and useful markers in an attempt to create a single tube ten color MFC panel that can be used during initial diagnosis and subsequently for MRD detection. Our findings were comparable with Thembare, et al. and Jain, et al. in CD73 and CD86 being the most effective markers in establishing LAIP at diagnosis in BALL cases. In this study, under-expression of CD81 was the next most frequent LAIP which was seen in a significant number of our cases at diagnosis. Muzaffar, et al. have studied the utility of CD81 in BALL MRD detection and have concluded that decreased CD81 expression is a sensitive and specific marker for residual disease even in a background of hematogones [21]. CD 81 has not been widely studied in India and our study proposes CD81 could be a useful marker to add to the MRD panel.
CD44 and CD58 were almost similar in terms of LAIP expression and hence any one of them could be used. In contrast to the above studies, CD123 seemed to be a less useful marker in this study and could be explained by lesser cases with hyperploidy [15] as we had a mixed population of pediatric and adult cases. CD9 on the other hand was expressed by almost all the cases; however, in terms of differential expression with respect to hematogones, it did not prove to be a very useful marker.
The greatest advantage with the theoretically proposed panel for MRD detection is the optimization of the resources which gets reduced to almost one-fourth of the entire cost involved (Rs.3,800/- (USD 48)). There are few limitations in this study and need to address these issues in near future with further prospective studies. First, we have not incorporated the study of stability of these markers following therapy and work is in progress in terms of the validation of these markers in the post-therapy identification of residual blasts using this single ten-color tube in our department. Second, the proposed single tube panel is lacking another pan B-cell marker, it is not possible to determine the effects of targeted treatment on immunophenotype with regard to the suggested panel if an anti-CD19 drug was given. Third, we had practical difficulty (non-availability) in picking up antibodies in the theoretically proposed panel with appropriate fluorochromes along with their respective clones to form the single tube panel which had 98% chances of picking more than 2 LAIPs. In order to strike the balance between these two issues, our newer chosen panel for the validation part (CD45, CD19, CD10, CD20, CD34, CD38, CD58, CD73, CD86 and CD123) will able to correctly identify >2 LAIPs in the 93% of cases analysed. The future prospective studies are required to validate our theoretically proposed panel.
To conclude, one of the greatest challenges and the first step in MRD detection starts with having the right panel of markers and understanding their expression pattern in the hematogones and residual blasts. Newer laboratories especially in resource constrained settings wanting to start MRD often requires both the cost effective and sensitive panel. In the light of our study and similar studies we propose a theoretical single ten-color tube composed of CD45, CD34, CD38, CD10, CD19, CD20, CD73, CD86, CD81 and CD44 for diagnosis as well as for tracking MRD in low- and middle-income countries like India.
Not applicable
Nil
Nil
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sujaya Mazumder, Prabhu Manivannan and Chandni Bhandary. The first draft of the manuscript was written by Sujaya Mazumder and all authors commented and corrected the previous versions of the manuscript. All authors read and approved the final manuscript.
Ethical approval was waived by the local Ethics Committee of the institute in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
We would like to thank our technical officer for flow cytometry laboratory Mrs. Sushya and Mrs. Manimegalai for the technical assistance in processing of all the samples in the study.
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
Citation: Mazumder S, Manivannan P, Bhandary C, Kar R, Kayal S, Basu D (2022) Proposed Single-Tube Ten-Color Antibody Panel to Optimize Resources for Measurable Residual Disease Detection in B-Acute Lymphoblastic Leukemia Based on Leukemia Associated Immunophenotype Evaluation at Diagnosis: A Single Center Experience from Southern India. J Leuk. 10:308
Received: 05-Aug-2022, Manuscript No. JLU-22-18715; Editor assigned: 10-Aug-2022, Pre QC No. JLU-22-18715; Reviewed: 26-Aug-2022, QC No. JLU-22-18715; Revised: 02-Sep-2022, Manuscript No. JLU-22-18715; Published: 12-Sep-2022 , DOI: 10.35248/2329-6917.22.10.308
Copyright: © 2022 Mazumder S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.