Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2023)Volume 14, Issue 2
Poly-L-Lactic Acid (PLLA) is a synthetic, biodegradable, resorbable and biocompatible polymer. Its use in the treatment of facial rejuvenation is already widely known, while its use to improve flaccidity and body contour has also been proposed, with reproducible and scientifically proven results. It is currently estimated that the greatest use of biostimulators in the Brazilian market is aimed at body treatments. One of the justifications for this behavior is the fact that Brazil is a tropical country, with a hot climate, in which body exposure and appreciation of body beauty are culturally evident.
Purpose: The purpose of this study is to suggest a restoration and application technique of PLLA, Rennova Elleva® (ER-Rennova Elleva®, Ghana R&D CO, LTD-South Korea) and Rennova Elleva X® (EXR-Rennova Elleva X®, Ghana R&D CO, LTD-South Korea), for the treatment of body flaccidity, through a simplified and reproducible method.
Methods: For body use, ER must be diluted in 20 ml of injectable water and 4 ml of lidocaine, forming a final solution of 24 ml that must be distributed across the superficial subcutaneous plane through 8 fan-shaped applications of 3 ml each, through 6 linear retrograde injections of 0.5 ml in each fan.
Results: With the restoration and application technique described, there is an improvement in the sagging and firmness of the skin, as well as in the appearance of the areas affected by cellulite.
Conclusion: This article has a methodology for the application of ER and EXR PLLA for the treatment of different body regions, their restoration, and a standardization of marking in 8 fans for the body region.
Body contouring; Collagen stimulator; Poly-L-Lactic Acid (PLLA); Practice guidelines; Skin laxity
According to ISAPS (International Society of Aesthetic Plastic Surgery), in the period from 1997 to the most recent available statistics (2021), an increase of almost 40% has been observed in aesthetic procedures considered minimally invasive, that is, there is a growing interest in non-surgical treatments. Access to these procedures is increasing among the population, as a result of their safety and cost-effectiveness. Additionally, these procedures present a recovery that, in most cases, does not require rest or absence from work [1].
The era of image and social media encourages the gradual search for aesthetic treatments, for both the face and the body, following the pulsating appeal of beauty and youth in modern times. Individuals show concern not only regarding the face, but also with the body, and the demand for body aesthetic procedures is also growing. It should be noted that, since Ancient Greece, the identification of ideal body proportions has been one of the most important subjects in the work of philosophers and artists such as Leonardo da Vinci [2].
Furthermore, the trend towards rejuvenating non-facial areas is justified by patients who acknowledge the disparity and stigma that arise between a treated face and untreated body areas [3].
Non-facial treatment areas include areas such as buttocks, thighs (internal, anterior, and posterior sides), abdomen, arms, neck, and décolletage. The most frequent complaints regarding these non-facial areas are skin flaccidity, cellulite, localized fat, cervical wrinkles, and cervical flaccidity [3].
With aging and, especially, frequent weight gain after the hormonal decline that occurs mainly around 40 years of age, the quality of tissue coverage worsens. There is a loss of structures that provide firmness and density to the skin, such as collagen and elastin, often resulting in sagging, worsening tissue texture, and adipose tissue accumulation. Thus, Poly-L-Lactic Acid (PLLA), by stimulating collagen production through its mechanism of action, is an excellent option for a minimally invasive body treatment to improve skin firmness [4,5].
Poly-L-Lactic Acid (PLA)-history
PLLA has been available for use on the market for over 20 years. It is considered an excellent biostimulator, although its use as a volumizer is also disseminated. In 1999, it was approved for use in Europe as a filler, under the trade name New-Fill® (Biotech Industry SA) [6].
In 2004, it was approved by the FDA for the treatment of HIVassociated lipoatrophy, under the name of Sculptra® (Dermik Laboratories, Sanofi Aventis, United States). In 2009, its use was expanded for aesthetic purposes in immunocompetent patients [7]. Since then, other countries have released its use and marketing for aesthetic purposes, and it is now used in 33 countries [4].
In Brazil, it has been available for marketing for 18 years (since 2005), with several studies being already available on its use, safety, and indications [5,8,9].
In 2021, a new PLLA was approved for aesthetic use in Brazil under the name Elleva, marketed by Rennova Elleva® (ER, Ghana R&D CO, LTD-South Korea), with the proposal to include in its vial 40% more microparticles produced by an exclusive technology named 3Homos+. It comprises a process of lyophilization of PLLA, being hermetically packed in a vacuum, and provides a quick restoration and a homogeneous suspension [9].
In 2022, with the growing interest in body treatments, a new presentation of ER was launched on the market, with the proposal to provide a package with three times more particles than the standard ER package, called Rennova Elleva X® (EXR, Ghana R&D CO, LTD-South Korea). EXR presents the ideal amount of PLLA for body application, which requires a greater amount of the product, being ideal for conventionally treating three body areas [10].
The following are considered body areas: Both buttocks, both medial compartment of the thighs, both compartment sides of the thighs, both posterior compartment of the thighs, both arms, and the abdomen. The decolletage and neck are considered a unit of body area.
Despite the convention of body areas and that one Elleva product is applied to treat one area, it is mandatory to individualize the treatment and consider using 2 or more vials per body area.
PLLA chemistry
It is a biodegradable, resorbable and biocompatible synthetic polymer. The particle size, ranging from 40 to 63 micrometers in diameter, is adequate to induce a good biocompatibility reaction with organic tissue (type II) [4], with sufficient dimensions so that they are not phagocytosed by macrophages and do not pass through the capillary wall. They are also suitable for application through 22G gauge needles or cannulas [11,12].
Mechanism of action
PLLA is, in its essence, a collagen biostimulator. After contact with tissues, a subclinical, controlled and desired inflammatory foreign body response primarily occurs. In the first 48 hours, there is a release of cytokines and monocytes, as well as recruitment of macrophages. From the second day onwards, giant cells are formed and the macrophages begin to secrete growth factors: PDGF-b and TGF-b, which act in the proliferation and differentiation of fibroblasts into myofibroblasts and are the ones that begin to secrete type I and type III collagen and hyaluronic acid from the third week onwards. Growth factors also act by preventing the degradation of the newly formed hyaluronic acid [11,13]
Within 3 to 4 weeks, around each microsphere there is formation of a dense and avascular collagen capsule that forms around the product. With time, there is a slow degradation of the material and a concomitant gradual neocollagenesis. Collagen deposition produced by the myofibroblast, stimulated by PLLA, promotes an evident increase in the thickness of the dermis over the weeks following application [14].
A skin ultrasound showed study an increase of 4 to 6 mm in the dermis after treatment with PLLA in patients with HIV-associated lipoatrophy [15,16].
After clinical application, there is a filling effect that lasts for up to 3 days, with complete absorption of the diluent: Sterile Double-Distilled Water (SDDW) and anesthetic. After these first 3 days, the Poly-L-Lactic Acid (PLLA) starts its neocollagenesis, which gradually produce the aesthetic effect, as the new collagen is deposited [14].
Recommendations for use with PLLA
It is important to emphasize that the biological response can become evident in the form of a “result” only after several weeks, and patient guidance in this regard greatly increases adherence to treatment. Another crucial issue is individual response, which varies according to age, the ability to stimulate collagen, diet, practice of exercise, skin quality, and health conditions in general [17].
The most exuberant neocollagenesis occurs after 6 months of treatment. The duration of the result for 25 months was proven in a North American study that showed the follow-up of patients 25 months after PLLA injection and found that the improvement remained for that time in 85% of the cases [15]. Degradation occurs by non-enzymatic hydrolysis into lactic acid monomers, which are transformed into CO2 and H2O, being then eliminated through respiration [14].
Reapplications are indicated after a period of at least 30 days, so that there is no overcorrection and in order to ensure that the tissue response time is followed. The product is applied sequentially so that there is a maintenance of the stimulus of the dermal microenvironment and, thus, the stimulus to the myofibroblast is maintained for some time prior to the new stimulus [14,18].
Experts believe that the number of sessions and vials used may vary according to the patient’s age and the clinical classification of the condition to be treated, such as flaccidity, cellulite, and localized volumetric depletion. The best known protocol for the use of PLLA is 3 applications with intervals of 45 days between them [15,17,18].
The product can be used as a single treatment or associated with technologies such as radiofrequency, aiming to optimize results with regard to flaccidity or cryolipolysis and even liposuction to reduce adipose tissue accumulation. When evaluating treatment response, muscle tone should be considered, as it influences the final results [16,17].
It is also necessary to evaluate aspects that may interfere with the final result: Quality of life, sedentary lifestyle, smoking, alcoholism, excessive sun exposure, dietary restrictions, menopause, or excessive exercise. Such factors may lead to a reduction in the therapeutic response and must be considered before initiating the treatment, and more sessions may be indicated to reach the desired result [16,17,18].
A detailed anamnesis should be carried out during the medical consultation, including information on previous body injections, personal history of comorbidities, current health status, portability of autoimmune diseases, and inflammatory or infectious conditions such as airway infections or herpes, in addition to the use of anticoagulants, life habits, allergies, and recent exposure to vaccines [19].
The pre-procedure photographs must be technical, in order to faithfully show the body conditions prior to the treatment. Reproducible tools should be used for photographing after treatment [20].
A consent term must be signed by the patient, demonstrating their express authorization to submit to the treatment. Patients must not have ingested medications that increase the risk of bleeding 10 days prior to the procedure. Body mass index, measurements of body circumference and weight, and classification of the degree of cellulite and flaccidity [19], are crucial parameters to be evaluated prior to starting treatment and comprise important diagnostic aids regarding the patient’s previous condition with regard to the degree of accumulation of adipose tissue in the regions to be treated.
Indications for ER use in body areas
PLLA has multiple and versatile applications for body areas.
1. Skin flaccidity resulting from aging and heavy weight loss.
2. Volumetric restoration resulting from the loss of body volumes, especially in the region of the buttocks.
3. Improved freshness, texture and shine of the skin on the face, neck, décolletage, and hands.
4. Treatment of cellulite and body flaccidity.
5. Volumetric restoration of depressions and body contour changes.
Contraindications
The contraindicated regions are already well known and must be considered for reasons inherent in the PLLA. General contraindications include cases of infection or local inflammatory process, active autoimmune diseases, collagenosis, pregnancy, presence of definitive fillers, and history of keloids or hypertrophic scars. Special attention should be given to collagen diseases that contraindicate the application of PLLA: Rheumatoid arthritis and its variants, Scleroatrophic Lupus, Scleroderma, Sjogren’s Syndrome, and Polymyositis/Dermatomyositis [17].
Body use
The use of PLLA on the body is already proposed in the scientific community, with reproducible and proven effective methods [21,22]. It can be used in regions such as the neck, decolletage, medial side of the arms, abdomen, buttocks, and thighs. Body applications should always be subdermal [19,21,23-26].
More specific treatments of grade 3 and 4 cellulite should be preceded by a light subcision with a blunt or cutter cannula so that the connective tissue adhesions can be undone before the application of PLLA [14].
For body surfaces, considering the best distribution of the product, the use of a cannula is good for application, providing safety and less risk of bruising.
Objective
Suggestion of a standard method for the restoration and application technique of PLLA, ER and EXR, for the treatment of body flaccidity, in order to optimize the distribution of the product and its performance, through a simplified and reproducible method.
ER restoration
ER is a sterile substance, composed of 210 mg of lyophilized PLLA microparticles, 132 mg of carboxymethylcellulose, and 178 mg of pyrogen-free mannitol. Its lyophilization process, hermetically vacuum-packed, provides fast restoration and homogeneous suspension [10].
ER restoration depends on the type of vial, ER or EXR, and the intended purpose.
For a volume effect, the product should be used in greater concentrations. For the effect of improving the quality of the skin, it should more diluted.
Below is a step-by-step process for ER restoration and hydration:
1. Wipe the vial cap with antiseptic.
2. With a 21G needle and a 20 ml disposable syringe, aspirate the desired amount of sterile distilled water and slowly adding it to the product vial.
3. Shake the vial vigorously for a few seconds, attaching the vial to the ER mixer 2.0 and press the button for adequate stirring for 60 seconds. If there is no mixer available, shake manually for 1 minute, with an interval of 10 minutes, for 1 hour.
4. After hydration in the mixer 2.0 or manually, aspirate the desired amounts into the syringes suitable for use.
Volume effect
Stock solution: 12 ml of ABD
Using a 3 ml syringe, aspirate 1.5 ml of the stock solution, followed by 0.5 ml of lidocaine.
Total: 8 syringes of 3 ml
Final volume: 16 ml
Skin quality improvement effect
Stock solution: 12 ml of ABD
Using a 3 ml syringe, aspirate 1.5 ml of the stock solution, followed by 1 ml of ABD and by 0.5 ml of lidocaine.
Total: 8 syringes of 3 ml
Final volume: 24 ml
EXR restoration
EXR is a sterile substance, composed of 630 mg of lyophilized PLLA microparticles, 396 mg of carboxymethylcellulose and 534 mg of apyrogenic mannitol. Its lyophilization process, hermetically vacuum-packed, provides fast restoration and a homogeneous suspension.
Volume effect
Stock solution: 36 ml of ABD
Using a 5 ml syringe, aspirate 4.5 ml of the stock solution, followed by 0.5 ml of lidocaine.
Total: 8 syringes of 5 ml
Final volume: 40 ml
Skin quality improvement effect
Stock solution: 36 ml of ABD
Using a 10 ml syringe, aspirate 4.5 ml of the stock solution, followed by 4 ml of ABD and 0.5 ml of lidocaine.
Total: 8 syringes of 9 ml
Final volume: 72 ml
Comments: The injection of the product must be carried out immediately after its restoration and homogenization by the mixer. The addition of lidocaine must be inside the respective syringes (3 ml, 5 ml, or 10 ml), just before the body injection.
Injection technique
The treated area must undergo asepsis, initially with 2% chlorhexidine and subsequently with 2% alcoholic chlorhexidine. The applicator should wait for it to dry before marking. Marking should be performed with the patient standing up, with the exception of the neck and décolletage. After marking, a new layer of alcoholic chlorhexidine should be applied just before the injection.
In the event that a topical anesthetic is to be used, it must be applied before the appointment and left to act for 30 minutes prior to injection. After removal, adequate antisepsis should be performed, and the region should be marked.
Injection plane: High subcutaneous layer (dermal patch).
Injection device: 21G x 70 mm cannula
Cannula entry point: 21G needle
Product distribution: It should be done through 8 fan-shaped applications, with the amount per fan and retrograde injection varying depending on the vial used (ER or EXR) and applied dilution.
The proposed technique is based on the distribution of EXR in 3 ml fans through 6 linear retrograde injections of 0.5 ml. The fans may be distributed over the body surface to be treated.
The suggestion of using fans with a cannula is based on the fact that the body surface is large and that the use of a cannula reduces patient discomfort, facilitating the distribution of the product.
Thus, one vial of ER is used to treat one large body area or two small body areas, while one vial of EXR is used to treat 3 large body areas or 6 small body areas.
Marking: Neck and décolletage (considered 2 small areas or 1 large area)
ER should be used in diluted form to improve skin quality.
With the patient lying in dorsal decubitus, draw a central longitudinal line (cervical midline) and 2 lateral longitudinal lines starting from the gnathion (Figure 1). Mark the entry points at the following positions:
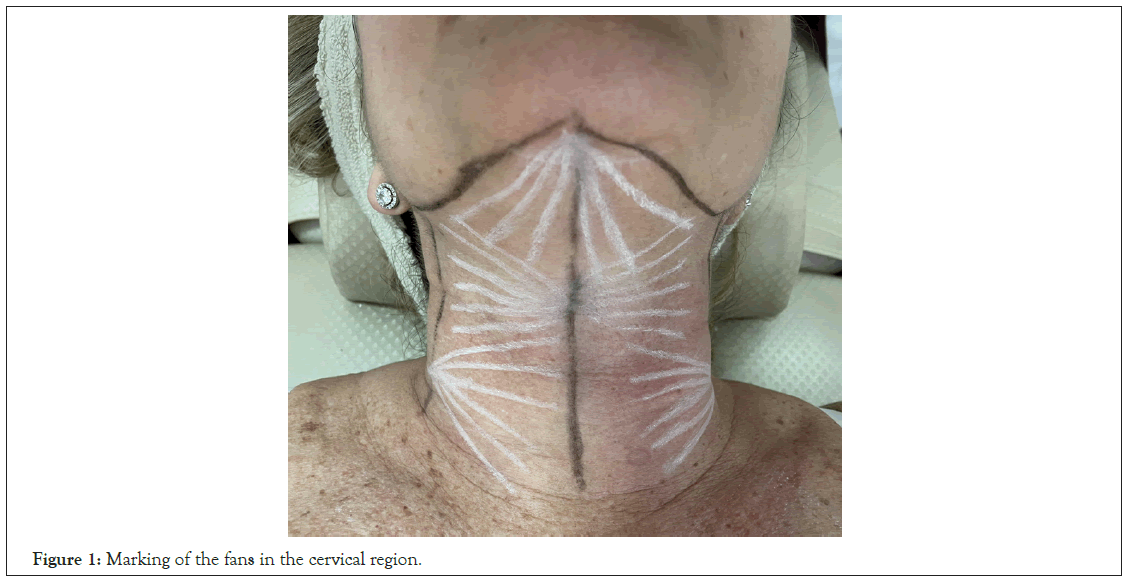
Figure 1: Marking of the fans in the cervical region.
• Gnathion: Draw a fan in the inferior direction of the mandibular gonion.
• 7 cm below the gnathion in the cervical midline: Draw 2 fans in opposite directions in the lateral direction.
• 10 cm below the gonion on the lateral cervical lines: Draw fans in a medial direction so as to fit in the lateral fans covering the entire cervical region.
• For the decolletage, 2 bilateral entry points starting from the most lateral point of the clavicles: Draw fans in the medial direction.
• One central entry point, 7 cm from the sternal notch: Draw an upper fan (Figure 2).
Figure 2: Marking of the décolletage area with 3 fans.
For the neck and decolletage, 8 fans of 3 ml.
Arms: In general, the inner side of the arms, the region with the greatest flaccidity, is treated.
For both arms, 1 vial of ER or 1/3 vial of EXR is recommended, with dilution to improve skin quality.
With the patient facing forward with arms abducted, draw a longitudinal line in the division between the biceps and triceps muscles, stretching from the most distal region of the arms, close to the elbows, to the most proximal region, close to the axillae. With the patient lying on her back, draw another longitudinal line dividing the upper arm musculature from the region of greater fat accumulation in the lower position. Join the ends of the lines by drawing an ellipse.
With the patient lying down, arms raised, arm and forearm joint flexed and hands overlapping behind the head, draw a longitudinal line that divides the marked region into two lateral halves. Draw 2 transversal lines crossing the median longitudinal line, 7 cm from the longitudinal end of the ellipse and from each other.
Entry points: Mark the entry points where the side lines intersect with the two cross lines. From each entry point, draw a fan for application of 3 ml of solution (Figure 3).
Figure 3: Marking of the arm with the ellipse, longitudinal line, and transversal lines.
Abdomen: With the patient standing, facing forward, draw a longitudinal line passing through the navel, dividing the abdomen into two lateral parts. At 10 cm laterally in relation to the central longitudinal line, draw 2 lateral longitudinal lines on each side of the abdomen.
From the longitudinal lines, draw 3 transversal lines, the first 10 cm from the umbilicus, a second line 7 cm below, and a third transversal line at the level of the navel.
Draw entry points at the intersection of the lines, so that there are 3 entry points on each lateral line of the abdomen and two points on the central line above the navel. From each entry point, draw fans of 3 ml each, with 6 paths and 0.5 ml per path (Figure 4).
Figure 4: Complete marking of the abdomen with 8 fans.
Gluteus: With the patient standing on her back, mark a straight horizontal line that passes through the highest point of the intergluteal fold. Following that, draw a vertical line on each side, dividing the buttock in half.
Mark the entry point on the vertical line, 7 cm below the upper horizontal line, and draw a fan in each of the 4 quadrants (superior medial, superior lateral, inferior medial, and inferior lateral).
In depression areas, cellulite grades 3 and 4, visible on the gluteus contraction maneuver, make a detachment with the cannula prior to the injection of ER (Figure 5).
Figure 5: Marking of the buttock area, with 6 retrograde injections in each quadrant.
Thigh-posterior compartment: With the patient standing on her back, draw alone across the infragluteal sulcus and a transversal line in the popliteal region. Draw two more equidistant transversal lines, dividing the thigh into three equal thirds. Draw a median longitudinal line dividing the thigh into two lateral halves and a lateral thigh line at its lateral limit at 7 cm to 10 cm laterally in relation to the median line.
Mark the entry points at the intersections of the transversal lines with the median line and draw fans upwards, totaling 3 fans. Then mark another entry point at the intersection between the lateral vertical line and the third transversal line, making a lateral fan (Figure 6).
Figure 6: Marking of the posterior compartment of the thigh.
Thigh-front side: With the patient standing, draw a longitudinal line from the front, dividing the anterior thigh into 2 lateral parts. Draw 4 horizontal lines starting from the lowest point of the vulva (Figure 7), dividing the thigh into 4 equal parts.
Figure 7: Marking of the anterior compartment of the thigh with 4 fans.
Mark 1 entry point at each intersection of the longitudinal line and the transversal lines, totaling 4 fans downwards, for each anterior compartment of the thigh.
Thigh-inner side: With the patient standing and facing forward, draw an oblique ellipse, running from the height of the body of the pubis to the medial surface of the proximal tibia.
With the patient lying down and the knees flexed and abducted, continue the marking delimiting the region of greatest accumulation of fat on the inner posterior surface of the thigh, closing the ellipse distally.
Draw a longitudinal line dividing the delimited region in half, into two lateral parts. Make 4 entry points on the longitudinal line, starting at the distal point of the ellipse and the subsequent points 7cm equidistant from each other. From each entry point, fan upwards (Figure 8).
Figure 8: Marking of the inner side of the thigh with 4 fans.
Post-procedure recommendation: Right after applying the product, a vigorous massage is performed for approximately 5 minutes, aiming to ensure adequate and uniform dispersion of the product across the treated region and to avoid visible accumulations on the surface of the skin. Massage with chlorhexidine 2% solution is recommended, thanks to its antiseptic effect and the fact that it facilitates sliding. It is strongly recommended that the patient maintain this massage at home 5 times a day, for 5 minutes and for 5 days straight [10]. Additionally, the patient should be advised to avoid sun exposure and exercise for 48 hours after the injections.
Collagen stimulators commonly used on the face are now extended to rejuvenating various parts of the body. PLLA has shown its efficiency and safety in improving flaccidity, volume, appearance of cellulite, skin quality and body contour.
For better results, combination with other procedures, such as radiofrequency, lasers and shock waves is recommended, bringing additional benefits mainly by improving the quality of the skin.
Our initial experience with ER, in the reconstitution and application technique described, showed good results in terms of improved skin firmness, buttock volume, as well as the appearance of areas affected by cellulite. More prospective studies are needed to better understand its action in these regions.
The safe and proven use of PLLA for over 20 years on the face has recently led to its use being encouraged for body treatments. Due to the knowledge of PLLA microparticles, which stimulate collagen production, the option of treating sagging in non-facial areas is a valid option, with a number of studies demonstrating its benefit.
With a new product on the Brazilian PLLA market, ER, there is a need to standardize its restoration and distribution in a way to facilitate its application and optimize its results. With that, through our experience with PLLA, we obtained a standard restoration and distribution method through 8 fans per region. We recommend the use with the cannula, thanks to its greater safety and homogeneous distribution.
Our suggestion allows the PLLA microparticles to be distributed throughout the body region chosen for treatment. It should be mentioned that we standardized the use of 210 mg of PLLA per body area, considering the neck and décolletage as one area. The individualization of each case allows the use of half or double this dose, the choice of the amount injected being up to the physician, always considering the patient’s needs and financial conditions.
In the event that the need is for volumetric replacement in the buttocks, Rennova® Elleva X may be diluted to 40 ml of final solution. A volume of 20 ml should be injected per gluteus, with 4 fans of 5 ml each, using a 5 ml syringe in each quadrant where volumetric replacement is required.
This article presents a methodology for applying PLLA-Rennova® Elleva and Elleva X for the neck, décolletage, arms, abdomen, buttocks, and thighs, as well as its restoration with sterile doubledistilled water and lidocaine, handling of the reconstituted product for better hydration of the PLLA particles, use of the 22G cannula, and a suggested standardization of the marking in 8 fans for each body region to be treated, through a simplified and reproducible method of application of the product.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Nogueira PL, de Morais Teodoro MRF (2023) Protocol for the Use of Poly-L-Lactic Acid (Elleva and Elleva X) for Skin Flaccidity in Body Areas. J Clin Exp Dermatol Res. 14:629.
Received: 17-Feb-2023, Manuscript No. JCEDR-23-21859; Editor assigned: 20-Feb-2023, Pre QC No. JCEDR-23-21859 (PQ); Reviewed: 06-Mar-2023, QC No. JCEDR-23-21859; Revised: 13-Mar-2023, Manuscript No. JCEDR-23-21859 (R); Published: 20-Mar-2023 , DOI: 10.35841/2155-9554.23.14.629
Copyright: © 2023 Nogueira PL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.