Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2018) Volume 8, Issue 4
The chemical evaluation of Jatropha curcas (Barbados nut) seeds was carried out to analyze and characterize the chemical composition of Jatropha curcas seeds for mineral content. Various analytical techniques have been used to breakdown the mineral and fat and protein content. The result of the proximate composition was shown to be moisture (4.00%), ash (6.00%), fat (40.00%), fibre (8.00%), protein (27.65%) and carbohydrate (18.35%). The ascorbic acid content was found to be 5.16%. The result of the elemental composition of Jatropha curcas seeds shows the presence of Na (54.03 ppm), Al (15.68 ppm), Mg (166.23 ppm), P (5.08 ppm), Ca (1.81 ppm), K (79.89 ppm), Fe (112.03 ppm), Pb (0.10 ppm), Zn (63.23 ppm) and Cd (0.04 ppm). This study revealed that Jatropha curcas seeds have low moisture, high fat and protein content. This suggests that J. curcas can be good source of minerals, if it is detoxified.
<Keywords: Jatropha curcas; Ascorbic acid; Biomass; Energy production
Jatropha curcas (Linnaeus) belongs to the family Euphorbiaceae and is closely related to other important cultivated plants like rubber tree and castor etc. Because of its indispensible benefits to mankind it was classified among the first plant in 1753 with a Botanical name “jatropha” from a Greek word Jatro “meaning Doctor” and pha “meaning nutrition”. The plant is believed to be an origin of South America and Africa but later spread to other continents of the world by the Portuguese settlers [1]. According to Mike, Jatropha has both male and female plants, which may produce different yields of nuts [2]. This plant is of different varieties which include, Cape Verde, Nicaragua, Ife-Nigeria, non-toxic Mexican varieties [3].
Dormancy is induced by fluctuations in rainfall and temperature/ light; nevertheless, not all trees respond simultaneously. It is a vigorous drought and pest-tolerant plant that can grow on barren and eroded lands under harsh climatic conditions. It is easily established and grows very quickly. In a hedge you may have branches without leaves beside ones full of green leaves. It is a vigorous drought and pesttolerant plant that can grow on barren and eroded lands under harsh climatic conditions. The Jatropha plant can reach a height up to 3-5 m and its seed yields ranges from 5 to 12 tons per hectare per year, after five years of growth. The life-span of Jatropha may fall between 35-50 years depending on the climatic condition [3]. The various images of Jatropha curcas plant and seeds are shown below in Figures 1 and 2:
The growing interest in Jatropha curcas as a biodiesel to help alleviate the energy crisis and generate income in rural areas of developing countries make people call it “miracle tree”, according to Mike [2]. It was found in research work on renewable energy that in Nigeria Jatropha is called several names by different tribes Binidazugu or lakwa- lakwa in Hausa, Oriokwu in Igbo, Lapalapa in Yoruba, Muaayiin Gbagyi, Kasha in Nupe, Ebe’bro in Yala [4]. Hence, this study is focused on the proximate analysis and elemental composition of Jatropha curcas seed to ascertain its usefulness in the production of energy.
Sample collection and preparation
Fruits of Jatropha curcas were obtained from Pankshin Local Government Area, of Plateau State of Nigeria, in June 2016. The seeds were then removed from the fruit, cracked and placed on the asbestos pad and dried in the oven at a temperature of 60-70°C. They were brought out and kept for air circulation. The dried sample was ground into powdered form and stored in an air tight container for further analysis.
Proximate analysis: Proximate analysis involves determination of moisture, ash, crude fibre, crude protein, fat and carbohydrate contents. Carbohydrates were calculated while ash, moisture, fats, fibre and crude protein were determined through methods described by (A.O.A.C.) [5].
Determination of moisture content (A.O.A.C.): The powdered sample of the seeds was weighed (5 g) into pre-weighed beaker and placed in an oven for six (6) hours at a temperature of 100°C to a constant weight. The loss in weight was expressed as a percentage of the initial weight. Thus, the different in weight indicates the amount of water contained in the sample.
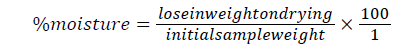
Weight of empty beaker (a1)=30.1 g
Weight of empty beaker+sample (a2)=35.1 g
Weight of empty beaker+dry sample (a3)=30.3 g
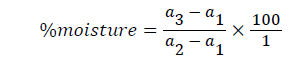
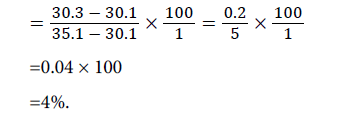
Determination of ash content (A.O.A.C.): The 5.0 g of the powdered sample was weighed into a pre-weighed labelled crucible and placed in the muffle furnace at a temperature of 50°C for 20 minutes. The furnace was allowed to cool before removing the crucible with its content. The crucible was later cooled in a desiccator and reweighed to get the ash content.
Weight of empty beaker (b1)=30.1 g
Weight of empty beaker+sample (b2)=35.1 g
Weight of empty beaker+dry ignited sample (b3)=30.4 g
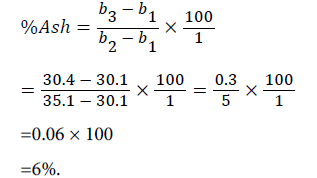
Determination of crude fat content (A.O.A.C.): The 5.0 g of the powdered sample was put into the soxhlet extractor thimble wrapped with a filter paper and plugged tightly with cotton wool. 150 ml of petroleum ether (bpt 60-80°C) was poured into 300 ml round bottom flask containing anti-bombings and the soxhlet extractor assembled. The sample was extracted for 4hrs until the extract become colourless. The extract was poured into a dried pre-weighed beaker and the thimble rinsed with a little quantity of petroleum ether back into the beaker. The beaker was heated on a steam bath to drive off the solvent. The extracted fat left in the beaker was dried in one desiccator and weighed.
The percentage crude fat was calculated as follows;
Weight of empty round bottom flask (c1)=23.3 g
Weight of round bottom flask+sample (c2)=28.3 g
Weight of round bottom flask+(dry fat), lipid (c3)=25.3 g
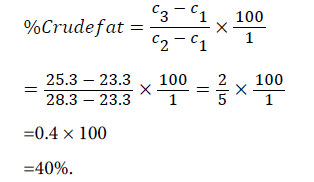
Determination of crude fibre content: The 5.0 g of powdered sample was put in a pre-weighed beaker. 50 ml of 1.25% H2SO4 solution was added and made up to 200 ml with distilled water and stirred. The mixture was heated with continuous stirring for thirty (30) minutes and allowed to cool and settle. Distilled water was added and allowed to settle then decanted, decantation was repeated for six (6) times consecutively to make the mixture acid free. 50 ml of 1.25% NaOH was added to 200 ml with distilled water in a beaker and heated for thirty (30) minutes with continuous stirring. It was cooled and allowed to settle. Distilled water was added and decanted for six (6) times consecutively. The mixture was filtered with filter paper and kept for forty-five (45) minutes for water to drain completely and the weight taken.
Weight of empty beaker (d1)=97.7 g
Weight of empty beaker (d3)=102.7 g
Weight of empty beaker+dry fibre (d3)=97.7 g
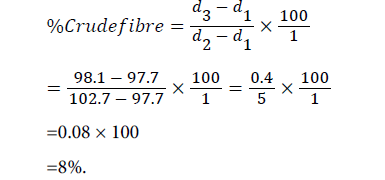
Determination of total carbohydrate content: This was obtained by taking each percentage value of protein, fat, fibre and ash content from the total dry matter.
Protein=27.65%
Fat=40.00%
Fibre=8.00%
Ash=6.00%
———
81.65%.
% Total carbohydrate content:
=100-81.65
=18.35%.
Determination of crude protein content (Modified Kjeldahl method): The analysis was carried out in three (3) stages, these were;
• The digestion stage.
• The distillation stage.
• The titration stage.
Digestion stage: The powdered sample was weighed out 5 g into a 250 ml kjeldahl flask. 2 g each of the kjeldahl catalysts (Copper Sulphate and Sodium Sulphate) were weighed into the kjeldahl flasks. Anti-bumping granule was added and 30 ml of concentrated Sulphuric acid was also added to the flask. The digestion flask was then placed on the heating mantle for an hour before being transferred to electric stove. The digestion process proceeds with occasional swirling until a clear solution was obtained. The clear solution was transferred into a 100 ml standard flask and made up to the mark with distilled water.
Distillation stage: The 10 ml of the digest was measured into the micro distillation apparatus. 12.5 ml of 1.25% NaOH was also added to the flask. A condenser was connected from the distillation apparatus to a volumetric flask containing 10ml of 5% boric acid and 2 drops of double indicator (methyl red and methyl blue). The distillate was collected in a flask and then titrated with 0.1 ml standard hydrochloric acid until a pale pink colour end point was obtained.
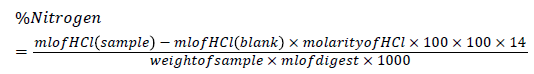
%Protein=%Nitrogen × %Protein factor
=4.424 × 6.25
=27.65%.
Determination of ascorbic acid content (Redox titration using iodine solution): The solution of iodine 5.0 × 10-3 M was prepared, 5 g of the powdered sample was weighed and made into a paste by further grinding with mortar and pestle. 100 ml of distilled water was then added to the paste. The solution was filtered with a filter paper. 10 ml of the filtrate was pipetted into a conical flask. It was than titrated with the iodine solution until a dark blue-black colour was obtained. The titration was repeated with further aliquots (10 ml) of sample solution until concordant results were obtained as shown in Table 1.
| Burette Readings | 1st (cm3) | 2nd (cm3) | 3rd (cm3) |
|---|---|---|---|
| Final | 5.8 | 25.7 | 43.6 |
| Initial | 0 | 20.4 | 37.9 |
| Volume of iodine used | 5.8 | 5.3 | 5.7 |
Table 1: Result of ascorbic acid content.
Avarage volume of Iodine used 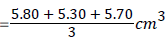
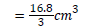
=5.60 cm3
Volume of blank titre=18.5 cm3
Volume of iodine used=5.60 cm3
Standard Ascorbic Acid in 10 ml of H2O=0.20 g/l
Weight of Sample=5 g
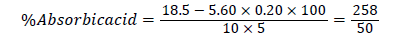
=5.16.
Elemental composition
The seeds were removed from the fruit, cracked and placed on the asbestos pad and dried in the oven at a temperature of 40°C. They were brought out and kept for air circulation. The dried sample was ground into powdered form and stored in an air tight container for further analysis. The powder mass was digested with some acids mixture (Perchloric acid, Sulphuric acid and Nitric acid), 10 ml of the digest was measured into a 250 ml flask and make-up to the mark with ionized water and sent to Akwa Ibom State University of Technology for AAS measurement for the following metals Na, Al, Mg, P, Ca, K, Fe, Pb, Zn and Cd using different cathode lamps for the element of interest to be analyzed. Some of the freshly chopped minute pieces were used in determining moisture content, ash content and for further analysis.
Proximate composition
The result of the proximate composition of Jatropha curcas seed is presented in Table 2.
| S/N | Parameters | Raw Values (%) |
|---|---|---|
| 1 | Moisture content | 4 |
| 2 | Ash content | 6 |
| 3 | Crude fat content | 40 |
| 4 | Crude Fibre content | 8 |
| 5 | Protein content | 27.65 |
| 6 | Total carbohydrate content | 18.35 |
Table 2: Result for the Proximate Composition of Jatropha curcas Seed.
The results of the analysis (Table 2) shows the moisture to be 4.00%, ash 6.00%, lipid 40.00%, Fibre 8.00%, protein 27.65% and carbohydrate 18.35%.
Moisture content: The result of the analysis (Table 2) shows the moisture content of the seed to be 4.00%. This value is lower than 22.00% reported by Magu et al. for Chrysophyllum cainito seed [6]. This reveals that this Jatropha curcas seed will have a very short shelf life compare to the seed of Chrysophyllum cainito . This is because the moisture content of seeds, fruits and vegetables is indicative of their shelf life. The higher the moisture content, the more susceptible the seeds, fruits, vegetable is to microbial attack and reduce its shelf life.
Ash content: The result of the analysis (Table 2) shows the ash content of the seed to be 5.03%. This is higher than 4.00% reported by Odoemelam, for seeds of African oil bean [7]. This composition shows that Jatropha curcas seed has a high content of minerals.
Fat content: Table 2 shows the result of the analysis for crude fat. The result obtained is 40.00%. This is quite high. Seeds with high lipid contents are usually compared with those of soya bean oil, locust bean and cotton seed; 19.10 g/100 g, 20.20 g/100 g and 14.05 g/100 g crude fat respectively. These are commercially exploited and classified as oil seeds. This makes Jatropha curcas a good fat content source for both industrial and commercial use [8].
Fibre content: The result analysis (Table 2) shows the fibre content of the seed to be 8.08%. This value is higher than 6.00% reported by Akintayo et al. for soybean seed [9]. Fibre helps in the maintenance of human health and has been known to reduce cholesterol level in the body [10].
Protein content: The result of the analysis (Table 2) shows the protein content of the seed to be 27.65%. This value is higher than 23.6% reported by Ayodele et al. for cowpea, the result however, suggests that Jatropha curcas seed could be used as an alternative source of protein supplement [8].
Carbohydrate content: The result of the analysis (Table 2) shows the carbohydrate content of the seed to be 18.35%. This value is lower compared with 60.0% reported by Ayodele et al. for cowpea [8]. However, this result revealed that Jatropha curcas seed is not a good source of carbohydrate.
Ascorbic acid content: The result of the analysis (Table 3) shows the ascorbic acid content of the seeds to be 5.16%. This value is low compared to 66.88% reported by Chinyere et al. for lagenaria seeds [10]. This implies that, Jatropha curcas seed is not a good source of ascorbic acid compared to Lagenaria sphaerica seed.
| Parameter | Value (Percentage %) |
|---|---|
| Ascorbic Acid | 5.16 |
Table 3: Showing the Ascorbic Acid Content of Jatropha curcas Seed (% w/w).
The level of Mg, Fe, K, Zn and Na are high while those of Cd, Pb, Ca, P and Al are much lower (Table 4)(Figure 3). The seed could therefore be referred to as a good source of Mg, Fe, K, Zn and Na. Although zinc is a heavy metal, it has been found to be of low toxicity to man except on prolonged consumption of large doses, which could result in some health complication such as fatigue, dizziness and neutropenia [11]. Zinc on the other hand is an essential component of a large number (>300) of enzymes participating in the synthesis and degradation of carbohydrates, lipids, and metabolism of other micro-nutrients. Zinc stabilizes the molecular structure of cellular components and membrane structures and helps to maintain cell and organ integrity [12]. Calcium is a major factor sustaining strong bones and plays a part in muscle contraction and relaxations, blood clotting, synaptic transmissions and absorption of vitamin B12 [12,13]. The relatively high content of calcium (1.81 0.02 MgKg-1 FW) in J. curcas suggests that it may not be good of therapeutic value in hypocalcaemic state like osteoporosis. Iron level of J. curcas (112.46 ± 1.70 MgKg-1 FW) was higher than the FAO/WHO (1988) recommended dietary allowance for males (1.3 mg/day) and female (2.94 mg/day) [14]. Iron has been reported as an essential trace metal that plays numerous biochemical roles in the body, including oxygen-binding haemoglobin and acts as an important catalytic center in many enzymes for example, the cytochrome. Iron is an important trace element in the human body [15,16]. It plays crucial roles in haemopoiesis, control of infection and cell mediated immunity. Sodium is an extracellular cation involved in the regulation of plasma volume, acid-base balance, and nerve and muscle contraction. High dietary sodium has been associated with hypertension. Magnesium plays a significant role in carbohydrate metabolism, nucleic acids and binding agents of cell walls [11]. The presence of these minerals contributes to its medicinal value [6,17,18]. This suggests that J. curcas can be good source of minerals, if it is detoxified.
| Sl No. | Mineral Composition | Composition (Mg/Kg-1 KW) |
|---|---|---|
| 1 | Sodium (Na) | 54.03 |
| 2 | Aluminium (Al) | 15.68 |
| 3 | Magnesium (Mg) | 166.23 |
| 4 | Phosphrous (P) | 5.08 |
| 5 | Calcium (Ca) | 1.81 |
| 6 | Potassium (K) | 79.89 |
| 7 | Iron (Fe) | 112.03 |
| 8 | Lead (Pb) | 0.1 |
| 9 | Zinc (Zn) | 63.23 |
| 10 | Cadnium (Cd) | 0.04 |
Table 4: Results for the mineral composition of Jatropha curcas seed.
The present study on the determination of proximate composition, ascorbic acid content and elemental composition of Jatropha curcas seed was carried out using standard analytical methods. The study revealed that there is a low concentration of moisture (4%), fibre (8%) and ascorbic acid content (5.16%). The carbohydrate and crude protein (18.35% and 27.65% respectively) of the seed is high. This is an indication that Jatropha curcas seed is not a good source of carbohydrate, however, the protein and fat extract can be used industrially, especially in the production of biodiesel.
The results of this investigation on the elemental composition of Jatropha curcas seeds show the presence of Na (54.03 ppm), Al (15.68 ppm), Mg (166.23 ppm), P (5.08 ppm), Ca (1.81 ppm), K (79.89 ppm), Fe (112.03 ppm), Pb (0.10 ppm), Zn (63.23 ppm) and Cd (0.04 ppm), this suggest that J. curcas seed’s oil has great potentials for human nutritional purposes, but due to the presence of some toxic substances this cannot be achieved at the moment. So, detoxification is recommended. J. curcas oil can be used as a source of oil for the production of soaps, paints and lubricants. Further studies are on how J. curcas seed and seed oil can be more useful in human nutrition in form of dietary supplements and possibly for livestock feed production.