Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Case Report - (2022)Volume 13, Issue 2
Purpose: Phacolytic glaucoma is a secondary open angle glaucoma that is associated with hypermature cataract. The purpose of this study is to report a rare complication of phacolytic glaucoma.
Observations: A 70-year-old female was referred to us for elevated intraocular pressure (IOP) in the left eye. On exam, her left eye vision was light perception and IOP was 56 mmHg. Anterior segment exam was significant for 3+ conjunctival injection, microcystic edema, impending bullae, stromal edema, and deep chamber with 3+ cells, iridonesis, and mature cataract. Ultrasound biomicroscopy (UBM) confirmed open angle and iridescent particles in the anterior chamber. The diagnosis was phacolytic glaucoma. Oral methazolamide was started and trabeculectomy was scheduled in 2 days. However, patient developed microbial keratitis on the day of surgery. Despite fortified topical antibiotics, the keratitis worsened and led to endophthalmitis with orbital involvement, requiring eventual evisceration. Cornea culture came back positive for Pseudomonas aeruginosa Patient’s condition was stabilized after starting broad spectrum IV antibiotics.
Conclusions and Importance: Prolonged corneal edema with disrupted corneal epithelium from the temporary damage of high IOP on endothelium predisposed the eye to Pseudomonas aeruginosa that normally cannot infect intact epithelium. Therefore, care should be taken in determining the timing of and proper antibiotics course prior to surgery.
Phacolytic glaucoma; Pseudomonas aeruginosa; Microbial keratitis
Phacolytic glaucoma is a type of secondary open angle glaucoma that is associated with hypermature cataract. As the lens mature, high molecular weight proteins can leak through the lens capsule to induce local macrophage activity [1,2]. The combination of protein and macrophages in the anterior chamber can lead to an obstructed trabecular meshwork, resulting in elevated Intraocular Pressure (IOP). Patients typically present with painful decreased vision, and on examination, acutely increased IOP, conjunctival injection, anterior chamber reaction without keratitic precipitates, and a mature cataract with possible anterior capsule wrinkle. Corneal changes include microcystic edema secondary to elevated eye pressure and stromal edema secondary to endothelial dysfunction [2]. In this report, we describe an unusual complication of phacolytic glaucoma that leads to Pseudomonas aeruginosa keratitis.
A 70-year-old female was initially referred to us for surgical evaluation by an outside facility for elevated IOP in the left eye. Three years ago, patient was diagnosed with primary open angle glaucoma and cataract in the left eye with recommendation for cataract surgery at the same outside facility. However, the surgery was cancelled due to uncontrolled high blood pressure and subsequent loss of follow-up. For the past 3 days, she noticed worsening blurry vision with acute pain in the left eye. She was initially diagnosed with elevated eye pressure in the left eye at the outside facility, and was put on Brimonidine BID OS, Cosopt BID OS, and Latanoprost QHS OS.
Upon examination, her left eye vision was Light Perception (LP), and IOP was 56 mmHg. Anterior segment exam was significant for 3+ conjunctival injection, microcystic edema, impending bullae, stromal edema, and deep chamber with 3+ cells, iridodonesis, and mature cataract. Given no view of the fundus, a B scan was performed showing an anterior vitreous opacity likely from overspill from the anterior chamber (Figure 1). A scan was performed and axial length was 24.5 mm. Ultrasound Biomicroscopy (UBM), performed under sterile condition, and confirmed an open angle with less than one quarter of peripheral anterior synechiae and dense anterior chamber reaction with iridescent particles (Figure 2). A diagnosis of phacolytic glaucoma of the left eye was made. Patient was started on oral Methazolamide 50 mg TID, Cyclogyl TID OS, and Erythromycin ointment BID OS for comfort. Patient was then scheduled to have combined cataract extraction with intraocular lens placement and trabeculectomy in 2 days.

Figure 1: B scan of the left eye showing dense cataract and anterior vitreous opacity, likely from anterior chamber overspill.
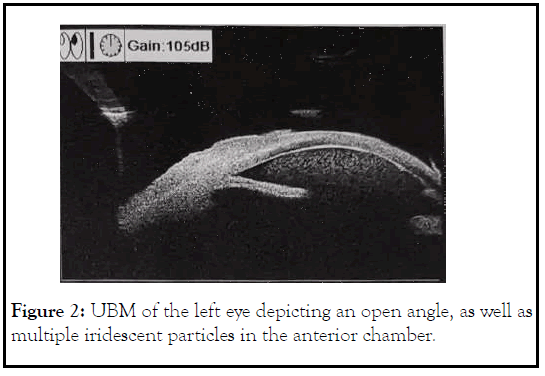
Figure 2: UBM of the left eye depicting an open angle, as well as multiple iridescent particles in the anterior chamber.
On the day of surgery, patient was noted to have corneal infiltrate in the left eye with positive fluorescein stain spanning the nasal half from 7 to 12 o’clock (Figure 3). Surgery was cancelled, and conjunctival and corneal cultures were taken. Patient was started on fortified vancomycin and tobramycin every 1 hour. Despite fortified antibiotics, patient’s clinical courses worsened 2 days later with progressive corneal infiltrate (Figure 4). Repeat B scan showed a vitreous opacity with choroidal thickening and detachment (Figure 5). CT scan was consistent with panophthalmitis with retrobulbar fat stranding. The gram stain showed gram negative microorganisms, so broad spectrum intravenous antibiotics was started, and the patient subsequently underwent evisceration of the left eye due to the extent of infection. On the following day, culture was positive for Pseudomonas aeruginosa. After confirming the microorganism was sensitive to ceftazidime, we increased intravenous ceftazidime to 2g three times a day. Patient was observed daily, and showed stable clinical condition following the evisceration. Patient was discharged 1 week after. Cytological examination of post eviscerated left eye revealed lens-protein laden macrophages, consistent with previous diagnosis of phacolytic glaucoma (Figure 6).
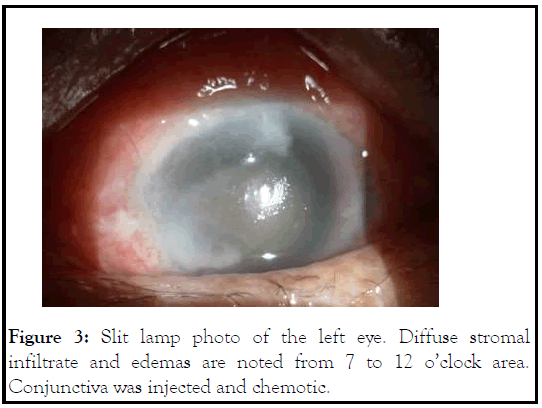
Figure 3: Slit lamp photo of the left eye. Diffuse stromal infiltrate and edemas are noted from 7 to 12 o’clock area. Conjunctiva was injected and chemotic.
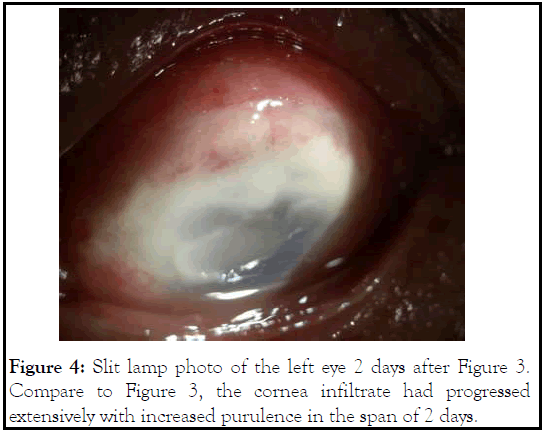
Figure 4: Slit lamp photo of the left eye 2 days after Figure 3. Compare to Figure 3, the cornea infiltrate had progressed extensively with increased purulence in the span of 2 days.
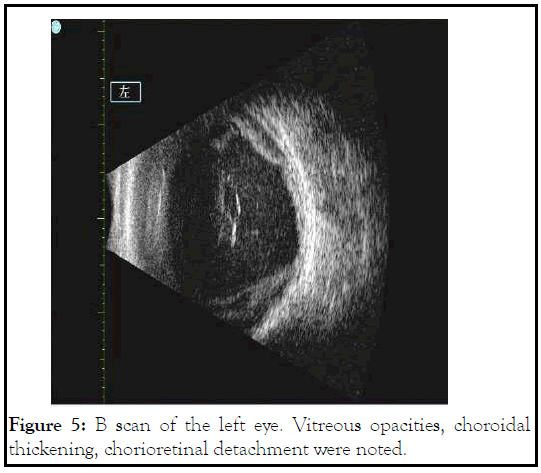
Figure 5: B scan of the left eye. Vitreous opacities, choroidal thickening, chorioretinal detachment were noted.
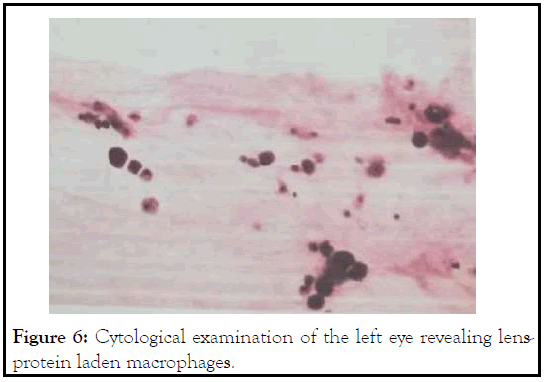
Figure 6: Cytological examination of the left eye revealing lensprotein laden macrophages.
Phacolytic glaucoma is an uncommon presentation that is more typically seen in rural or developing areas because of its onset associated with mature cataracts outside of traumatic capsule rupture. Typical symptoms of eye pain, photophobia, reduced vision, eye redness are characteristic but non-specific. On exam, the involved eye has elevated IOP, conjunctival injection, corneal edema if not chronically compensated, iridescent bodies in anterior chamber, and hypermature cataract. Complications of phacolytic glaucoma are usually related to damage to the optic nerve from elevated IOP. In this case, the complication was a microbial keratitis that unfortunately led to endophthalmitis. We hypothesize that prolonged corneal edema with disrupted corneal epithelium from the temporary damage of high IOP on endothelium predisposed the eye to an opportunistic bacterium that normally cannot infect intact epithelium.
Pseudomonas species, Staphylococcus aureus, and Streptococcus pneumonia are some of the most common bacteria grown in culture from microbial keratitis [3,4]. Pseudomonas aeruginosa is known to cause corneal and scleral melting within days due to a combination of its own virulence factors and inflammatory host response. Intact corneal epithelium prevents Pseudomonas infection as its pili only attach to injured epithelial cells and exposed stroma. Once adhered, stromal invasion occurs within hours causing diffuse stromal edema and inflammation involving the whole width and depth of the cornea. Corneal and scleral melt then follows within the next 48-96 hours, mediated by pathogen virulence factors such as exotoxins exoU and exoS, elastase LasB, and proteases as well as by host recruitment of polymorphonuclear leukocytes and activation of matrix metalloproteinases [3,5,6]. There is considerable risk of perforation due to these two processes even with adequate antibiotic treatment.
The most common risk factor for bacterial keratitis is contact lens use, however other risk factors include corneal trauma, immunosuppressive medications such as topical steroids, postocular surgery, prolonged usage of topical antibiotics, and ocular surface disease [3,4,7-9]. Ocular surface disease includes exposure keratopathy, history of herpetic infection, and bullous keratopathy. In large institutional reviews of bacterial keratitis cases, the percentage of associated keratopathies range from 4.7%-21% of cases [4,8,10]. Specifically for cases of bullous keratopathy, prolonged duration of untreated or unresolved edema was found to be an independent risk factor and prophylactic antibiotic use did not prevent future development of corneal ulcers [10].
History of glaucoma has been associated with microbial keratitis in elderly population needing eventual evisceration and enucleation [11]. The author postulated that epithelial irregularities, compounded by ocular surface damage from preserved glaucoma medication, likely contribute to the association between elevated eye pressure and microbial keratitis. In our patient, the acute rise in IOP likely damages the endothelial function, as can be seen in earlier study [12]. Endothelial dysfunction then leads to corneal edema, as well as microcystic edema and possible epithelial defect. This compromises the integrity of the epithelium, providing substrates for Pseudomonas attachment and subsequent infection.
The source of the corneal infection is very important. While we practiced sterile technique in acquiring diagnostic imaging like UBM, we could not be certain of patient’s hygiene at home. One potential source could stem from patient’s poor technique in instilling eye drop, where the contaminated surface of the eye drop bottle might have inadvertently touched the compromised cornea leading to infection. Other less likely source to consider including skin contamination with eye rubbing behaviors.
In this report, we described a case of bacterial keratitis due to Pseudomonas aeruginosa as a secondary complication from phacolytic glaucoma. In a review of the literature, there have been three cases of the reverse scenario in which bacterial keratitis was the initial diagnosis before further work-up demonstrated phacolytic glaucoma as the final diagnosis after surgical intervention and pathology report. In Agarwal’s study, their case initially presented to them with cornea opacification and vitreous opacity concerning for microbial keratitis and endophthalmitis. After surgical control of IOP from lens removal and anterior chamber paracentesis, the cornea status improved and phacolytic glaucoma was the final diagnosis [13]. In Dhingra’s study, their case exam was significant for diffuse cornea edema, conjunctival injection, and anterior chamber with white particles in addition to ultrasound biomicroscopy confirmed an irregular lens complex [14]. In Sahu’s study, a dislocated lens fragment was found adherent to the corneal endothelium causing an appearance of a deep corneal infiltrate suggesting bacterial keratitis but combining it with an intense anterior chamber reaction and elevated IOP is actually more consistent with phacolytic glaucoma [15]. Contrary to cases above, our patient had an initial diagnosis of phacolytic glaucoma that led to rapid progression of tissue destruction from secondary Pseudomonas keratitis.
In our case, we believe elevated IOP and pro-inflammatory anterior chamber environment temporally lead to endothelial dysfunction, resulting in cornea edema with disrupted ocular surface. This provides substrates for Pseudomonas attachment and subsequent stromal infection and melting. An argument could be made for operating earlier to avoid such complication. Instead, we waited two days prior to surgical management. We believe it is the right course because preoperatively lowering the IOP, as well as trying to control anterior chamber inflammation, can improve operative success and making operation safer through decrease chance of suprachoroidal hemorrhage and an improved corneal view. Furthermore, institutional studies show that phacolytic glaucoma patients can have symptoms for many days prior to seeking medical care [16-18], and there can be a few days in-between time of diagnosis and surgery [19]. Therefore, cautiously waiting a couple days to improve IOP can improve the safety of surgery without causing excessive delay in care.
In this case, we want to highlight that while optimizing IOP prior to cataract surgery, phacolytic glaucoma has the potential to cause microbial keratitis through disrupted ocular surface from elevated IOP. Therefore, care should be taken in determining timing of surgery and proper antibiotics course prior to IOP control.
Patient consent
The patient described herein consented to publication of the case in writing.
Funding
No funding or grant support.
Financial disclosures
The following authors have no financial disclosures: JY, JL, XW, XY, EC, JN, XL
Authorship
All authors attest that they meet the current ICMJE and Journal of Clinical and Experimental Ophthalmology criteria for Authorship.
Acknowledgments
We would like to thank Jiun Do for his support of the case.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Yu J, Liu J, Wang X, Yang X, Chan E, Nunez JR, et al. (2022) Pseudomonas Keratitis from Phacolytic Glaucoma. J Clin Exp Ophthalmol. 13:909.
Received: 27-Dec-2021, Manuscript No. JCEO-22-15232; Editor assigned: 29-Dec-2021, Pre QC No. JCEO-22-15232 (PQ); Reviewed: 12-Jan-2022, QC No. JCEO-22-15232; Revised: 17-Jan-2022, Manuscript No. JCEO-22-15232 (R); Published: 24-Jan-2022 , DOI: 10.35248/2155-9570.22.13.909
Copyright: © 2022 Yu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.