Chemotherapy: Open Access
Open Access
ISSN: 2167-7700
ISSN: 2167-7700
Research Article - (2015) Volume 4, Issue 1
Keywords: Coumarin, Quantitative structure activity relationship, 2D Descriptors, 17?-HSD3 inhibitors,Prostate cancer
Prostate cancer (PCa) is the most enigmatic of the common solid malignancies. Second only to lung cancer as a killer of men beyond middle age, it warrants more attention than it currently receives from governments, researchers and the general public worldwide [1]. Prostate cancer is the most frequently diagnosed cancer in men. According to the American Cancer Society, an estimated 192,280 men were diagnosed with prostate cancer in the US during 2009. With an estimated 27,360 deaths in 2009, prostate cancer is the second-leading cause of cancer death in men [2]. The human prostate is a hormonesensitive organ that depends on androgens for growth and development. The regulation of androgen biosynthesis or its action on the androgen receptor is central to the management of prostate cancer [3]. The early stages of prostate cancer tumor growth are androgen dependent and respond well to androgen ablation, [4-8] either via surgical castration or by chemical castration with a luteinizing hormone releasing hormone agonist in combination with an AR antagonist, such as bicalutamide. Recent evidence from both preclinical and clinical studies is consistent with the importance of reactivation of AR signaling in a majority of castrate-resistant prostate tumors [4-8]. 17β-Hydroxysteroid dehydrogenases are oxido reductases, which play a key role in estrogen and androgen steroid metabolism by catalyzing final steps of the steroid biosynthesis. The crucial role of estrogens and androgens in the genesis and development of hormone dependent diseases is well recognized [9]. Recent second-generation AR antagonists have been designed that retain antagonism in over-expressing cell lines, and among these agents enzalutamide [10] has recently successfully met efficacy criteria in a large Phase III clinical trial [11]. Quantitative structure-activity relationship (QSAR) is one of the most important applications of chemometrics, giving information useful for the design of new compounds acting on a specific target. QSAR attempts to find a consistent relationship between biological activity or toxicity and molecular properties. Thus, QSAR models can be used to predict the activity of new compounds [12]. Considering the recent interest in 17β-HSD3 inhibitors and for progression of design and development of such inhibitors, a QSAR investigation of the aforementioned series is carried out using two dimensional (2D) molecular descriptors. The present study aimed to establish relationship 17β-HSD3 inhibitors for structurally related 3- and 4-substituted 7-hydroxycoumarins derivatives and the physicochemical descriptors in quantitative terms. The statistically validated two dimensional quantitative structure activity relationship (2D QSAR) model was obtained through multiple linear regression (MLR) analysis method using Vlife molecular design suits (MDS)
Experimental Section
Dataset and biological activity
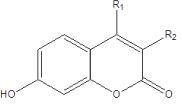
The 17β-HSD3 inhibitors activity was taken from the reported work [13]. We have converted the biological activity values [IC50 (μM)] reported in the literature to -log scale and subsequently used as the response variable for the QSAR analysis. The structures and in vitro activity of thirty five 3- and 4-substituted 7-hydroxycoumarins derivatives used in this study are listed in Table 1.
| S. No | R1 | R2 | IC50 | pIC50 | Pred. | Res. |
|---|---|---|---|---|---|---|
| 1 | (CH2)2-Phenyl | H | 0.02 | 7.698 | 7.671 | 0.02 |
| 2 | CH2O-Phenyl | H | 10 | 5 | 5.154 | -0.15 |
| 3 | CH2S-Phenyl | H | 0.093 | 7.031 | 7.251 | -0.22 |
| 4* | CH2NH-Phenyl | H | 4 | 5.397 | 5.241 | 0.15 |
| 5 | CH2NMe-Pheny | H | 6 | 5.221 | 5.286 | -0.06 |
| 6 | CH2S-2-Pyridiyl | H | 0.003 | 8.522 | 8.573 | -0.05 |
| 7 | CH2S-4-Pyridyl | H | 5 | 5.301 | 5.219 | 0.08 |
| 8 | CH2S-2-Pyrimidinyl | H | 1 | 6 | 5.877 | 0.12 |
| 9* | CH2S-2-Thienyl | H | 0.42 | 6.376 | 6.297 | 0.07 |
| 10 | CH2S-1,3,4-Thiadiazol-2-yl | H | 0.2 | 6.698 | 6.568 | 0.13 |
| 11 | CH2S-2-Thiazolyl | H | 0.01 | 8 | 8.143 | -0.14 |
| 12 | CH2S-2-Thiazolidinyl | H | 0.096 | 7.017 | 7.166 | -0.14 |
| 13 | CH2S-1-Methyl-2-imidazolyl | H | 0.091 | 6.04 | 6.233 | -0.19 |
| 14 | CH2S-5-Nitro-2-pyridyl | H | 0.088 | 6.055 | 5.944 | 0.11 |
| 15* | CH2S-5-Trifluoromethyl-2-pyridyl | H | 0.23 | 6.638 | 6.559 | 0.07 |
| 16 | CH2S-6-Methyl-2-pyridyl | H | 0.0015 | 8.823 | 8.736 | 0.08 |
| 17 | (CH2)2-6-Methyl-2-pyridyl | H | 0.008 | 8.096 | 8.141 | -0.04 |
| 18 | (CH2)2O-6-Methyl-2-pyridyl | H | 1 | 6 | 6.115 | -0.11 |
| 19 | CH2S-6-Methyl-2-pyridyl | F | 0.027 | 7.568 | 7.403 | 0.16 |
| 20 | CH2S-6-Methyl-2-pyridyl | Cl | 0.047 | 7.327 | 7.203 | 0.12 |
| 21* | CF3 | CH2S-2-pyridyl | 0.32 | 6.494 | 6.388 | 0.1 |
| 22 | Me | H | 1 | 6 | 6.081 | -0.08 |
| 23 | Et | H | 0.1 | 7 | 6.882 | 0.11 |
| 24 | n-Pr | H | 0.059 | 7.229 | 7.153 | 0.07 |
| 25 | CF3 | H | 0.19 | 6.721 | 6.764 | -0.04 |
| 26* | CH2OMe | H | 5 | 5.301 | 5.208 | 0.09 |
| 27 | Ph | H | 1 | 6 | 6.093 | -0.09 |
| 28 | CH2Ph | H | 10 | 5 | 5.192 | -0.19 |
| 29 | (CH2)2Ph | H | 0.02 | 7.698 | 7.71 | -0.01 |
| 30* | H | Me | 1 | 6 | 6.143 | -0.14 |
| 31 | Me | Me | 0.21 | 6.677 | 6.641 | 0.03 |
| 32 | Me | n-Pr | 0.24 | 6.619 | 6.59 | 0.02 |
| 33* | Me | CH2Ph | 0.2 | 6.698 | 6.726 | -0.02 |
| 34 | CF3 | CH2Ph | 0.03 | 7.522 | 7.452 | 0.07 |
| 35 | Me | F | 1 | 6 | 6.079 | -0.07 |
| 36 | Me | Cl | 0.1 | 7 | 6.945 | 0.05 |
| 37 | Me | CN | 1 | 6 | 6.119 | -0.11 |
Table 1: Structures and activities of 3- and 4-substituted 7-hydroxycoumarins as 17β-HSD3 inhibitors
A statistical subset selection was made using sphere exclusion algorithm [14] method to select a balanced and chemically diverse test set. The training set of 28 molecules was used to adjust the parameters of the models, and the rest of molecules were used to evaluate models prediction ability. The QSAR models were generated using a training set of twenty eight molecules and remaining seven compounds as a test set (Table 1 marked with asterisk) for validating the quality of the models.
Generation of molecular descriptors
All the molecular modeling and statistical analysis was completed using Molecular Design Suite TM 3.5, 2008,) software [15]. The structures of the compounds were sketched using molecular sketching facilities provided in the modeling environment of Vlife MDS. Energy minimizations were performed using Merck molecular force field (MMFF) and MMFF charge [16] followed by considering distancedependent dielectric constant of 1.0.
In our study, only 211 chemical descriptors were eventually used (after deleting these bookkeeping descriptors as well as those with zero value or zero variance). In this study to calculate AI descriptors, we have used following attributes, 2 (double bonded atom), 3 (triple bonded atom), C, N, O, S, H, F, Cl, Br and I and the distance range of 0–7. The program computes the best model based on squared correlation coefficient r2, crossed validated q2, F test, and pred_r2. A value of pred_r2 greater than 0.5 indicates the good predictive capacity of the QSAR model. The squared correlation coefficient (or coefficient of multiple determination) r2, is a relative measure of quality of fitness by the regression equation.
Correlations between different 17β-HSD3 inhibitors activities and calculated variables were established through multiple linear regressions using the method of least squares. Statistically significant QSAR model generated for activity data are as follow: pIC50=0.5809 ( ± 0.1377) T_2_Cl_1+0.4599 ( ± 0.0656) SdsCHcount-0.4644 ( ± 0.0937) SdssS (sulfone) count +0.6284 ( ± 0.1433) T_C_F_1. Ntraining=28, Ntest=7, r2=0.8291, q2=0.7455, F test=40.298 r2_se=0.3177, q2_ se=0.4936, pred_r2=0.7588.
2D QSAR model shows good correlation between 17β-HSD3 inhibitory activity of the molecules and number of SdsCHcount, SdssS (sulfone) count, T_2_Cl_1 and number of T_C_F_1. SdsCHcount (total number of –CH group connected with one double and one single bond) plays most important role in determining 17β-HSD3 inhibitory activity, which mainly indicates the relationship with reference to variation at R2 position. The positive correlation of the descriptor with the 17β-HSD3 inhibitory activity indicates that increase in the number of –CH group will increase activity of the title compounds. Other T_C_F_1 indicates number of carbon atom (single, double or triple bonded) separated from fluorine by single bond in a molecule. The descriptor is positively correlated with the biological activity in the model. So, it may be indicate that increase in the number of fluorine like in the molecules is conducive for the 17β- HSD3 inhibitory activity. Furthermore, the finding also suggests that increase in the fluorine group is detrimental to 17β-HSD3 inhibitory potency. Model has been obtained with correlation coefficient (r2)=0.8291 and the model explains about 82% variance in 17β-HSD3 inhibitory activity exhibited by 3- and 4-substituted 7- hydroxycoumarins derivatives. In this procedure high cross validated r2 (q2=0.7455) value, reflect the good internal predictive power of the model. The descriptor T_2_Cl_1, count of number of double bond separated from chlorine atom by single bond distances in a molecule suggesting that the presence of substituents with chlorine on the substituted 7-hydroxycoumarins at the 3rd and 4th position with will lead to an increase in activity. Such positive effect indicates that the 17β-HSD3 inhibitory activity was increase with the presence of chlorine and fluorine compounds such as compounds 13,17,30 and 35. Lastly descriptor SdssS (sulfone) count signifies the total number of sulphone group connected with two single and one double bond emphasizing and negative contribution of this descriptor in the QSAR model demonstrates that increased sulphone group branching on the substituents on 7-hydroxycoumarins nucleus will reduce to 17β-HSD3 inhibitory activity. As shown in Table 1, the predicted activity using developed QSAR equation was in close agreement with the reported activity. The contribution chart of selected descriptors are represented in Figure 1a and plots of predicted vs. observed values of pIC50 are shown in Figure 1b.
We have developed quantitative structure–activity relationship (QSAR) models for 17β-hydroxysteroid dehydrogenase inhibitors for potential treatment of prostate cancer of substituted 7- hydroxycoumarins derivative. This method utilizes multiple descriptors such as molecular connectivity indices, which are derived from two-dimensional molecular topology. After splitting the datasets, the respective descriptors were selected from pool of descriptors using the multiple linear regression method. By this method the most relevant descriptors were selected to build the model. These investigations will further help in rationalizing the design of 17β- HSD3 inhibitory activity molecules.
The author wishes to express gratitude to V-life Science Technologies Pvt. Ltd for providing the trial version software for the study.