Journal of Applied Pharmacy
Open Access
ISSN: 1920-4159
ISSN: 1920-4159
Research Article - (2021)Volume 13, Issue 11
Folklore herbal practioners of Cholistan desert claim Corchorus depressus Linn. (Tiliaceae) treat diabetes pain, fever, gonorrhea, treachery troubles, general weakness and sexual dysfunction. The aim of this study was to isolate α-glucosidase and anti-urease inhibitors and to check the α-glucosidase and anti-urease seasonal in three different time periods. In vitro α-glucosidase and anti-urease assays were carried out and in vivo anti-diabetic activity of Corchorus depressus Linn. Was studied on alloxan induced diabetic rats to show its traditional use. The fractions CD-13, CD-14, CD-15 and CD-20 showed 4.31 ± 0.07, 6.32 ± 0.08, 15.37 ± 0.13 and 12.42 ± 0.13 μg/mL with respect to Acarbose with IC50 37.38 ± 0.12 μM. The fraction CD-M7 and CD-J9 extracts were found active against anti-urease activity with IC50 1.63 ± 0.08 and 2.42 ± 0.07 μg/mL, respectively, as compared to thiourea with IC50 21.46 ± 0.13 μM. CDM7 and CD-J9 extracts were found active with IC50 1.63 ± 0.08 and 2.42 ± 0.07 μg/mL, respectively, as compared to thiourea with IC50 21.46 ± 0.13 μM. The most potent fraction CD-14 CDB was subjected to GC-MS analysis which resulted in isolation of metabolites as given in Table 1. Our results validate the traditional use of Corchorus depressus for traditional therapeutic potential in treating diabetes and ulcer diseases. So it was concluded from the following discussion that non-polar CD fractions (upto 20% EtOAc/Pet. ether) showed potent IC50 values for α-glucosidase inhibition but CD polar (water extracts) showed remarkable IC50 values for anti-urease inhibition.
Corchorus depressus; Anti-diabetic activity; Qualitative study
Diabetes mellitus is an epidemic disease all over the world. It is a metabolic disease that results in chronic hyperglycemia due to damage of pancreatic β-cells, lack of insulin secretion, insulin action or both [1]. The diabetes causing factors includes obesity, energy rich diet, sedentary lifestyle, blurred vision, frequent urination, sudden weight loss, increased thirst, hunger and sometimes fatigue [2]. Diabetes mellitus prevalence is increasing day by day and will be expected to increase by 5.4% in 2025 [3].
Corchorus depressus Linn., locally known as “Baphuli”, belonging to family Tiliaceae is mostly found in Pakistan, Arabia, India, Afghanistan. Pain, fever, gonorrhea, treachery troubles, general weakness, diabetes, gastric diseases, sexual dysfunction can be treated by it [4].
Hence, folklore importance of Corchorus depressus Linn., has prompted us to scientifically investigate its potential for diabetes and anti-urease activities. Our aim wasto develop scientific basis for the traditional ethnobotanical uses of Cholistani plants for diabetes, for the evaluation of anti-hyperglycemic effects of selected plant on animals and to check the anti-diabetic and anti-urease seasonal variations of various extracts and fractions of this plant in three different seasons (September 2015, January 2016 and May 2016).
From an extensive literature search it was observed that, no work has been reported in perspective of diabetes and anti-urease activities of this plant. However, C. olitorius and C. capsularis showed better anti-diabetic and anti-inflammatory activities Certain biological activities of this genus includes anti-fungal, analgesic, anti-bacterial and anti-pyretic ACE, anti-malarial, anti-anthelmintic [5]. Jute was reported for aging resistance of bio-epoxy jute-basalt hybrid composites as novel multi-layer structures for cladding [6] (Figure 1).
Figure 1: GC-MS analysis.
The chemicals used in this research were purchased from different sources, i.e. Alloxan was purchased from Acros Organics (USA), sodium chloride, sodium hydroxide, citric acid monohydrate, potassium hydroxide, benzene, ether, sulphuric acid, hydrochloric acid, chloroform, ethanol and methanol were all of analytical grade and were purchased from Merck (Germany).
Acarbose, thiourea, PNPG, methanol, ethanol, pet ether, dichloromethane, pyridine, ethyl-acetate, chloroform, diethyl amine were purchased from Sigma-Aldrich, Merk and Flukacompanies. NaOH, Na2CO3, HCl, Na2PO4, KH2PO4, DMSO were of analytical grade.
Instrumentation
The instruments used were rotary evaporator heating bath, recirculating chiller and pump (Buchi), vacuum pump, spectrophotometer (Shimadzo, Japan), HT Bio, EZTek96microplate reader, EZ-Fit Enzyme Kinetics software (Perrella Scientific Inc. Amherst, USA), Power Lab data acquisition system (AD instruments, Sydney, Australia) and Glucometer (Accu-check performa, Roche, Germany). Vortex mixture of HeidolphReax Top was used to make dilutions and to dissolve the crude extracts. The glassware used was made of pyrex. Shimadzuanalytical weighing balance was used to weigh the amount in milli-grams.
Plant collection and extraction
The whole plant of Corchorus depressus Linn. 20.5 kg along with roots had been collected from ChakLehaar on 14th May, 2018, Punjab, Pakistan. The plant specimen had been identified by Taxonomist, Mr. Muhammad Waris, Cholistan Institute of Desert Studies (CIDS), The Islamia University of Bahawalpur, Pakistan. The plant specimen (Voucher No.3473/CIDS/IUB) had been stored in the herbarium of CIDS for future reference.
After all, the plant was dried and marcerated in 20 lit of methanol for one week, the filtrate was evaporated, under high vacuum in a rotary evaporator to get 81 g crude methanolic extract of C. depressus Linn.
Biological activities
In vitro α-glucosidase inhibition activity: α-Glucosidase assay was carried out with slight modification as performed by Fiore V, et al. [7]. The assay contained 50 mM phosphate buffer pH 6.8 (70 μl), 0.5 mM test compound (10 μl), enzyme solution 0.02 Units (10 μl) to make a total volume of 100 μl. 10 μl of substrate PNPG was used to start the reaction. All the contents had been mixed up properly, pre-incubated for 10 minutes at 370C and pre reading was taken at 400 nm. Yellow colour absorbance took place indicates the formation of p-Nitrophenol. Epoch (Bio Tek, USA) 96-well microplate reader was used to calculate the absorbance at 400 nm. Acarbose was used as a positive control. The experiments had been carried out in triplicates.
The fractions CD-1 to CD-24 were subjected to α-glucosidase inhibition assay. Acarbose was used as a control with IC50 value 37.38 ± 0.12 μM.
Effects of seasonal variations on anti-urease inhibition activity of C. depressus Linn: The plant C. depressus was collected in three different periods (September 2015, January, 2016 and May, 2016). The 50 g of the plant material of all the periods was soaked in 250 ml n-hexane for 3 days, separately. The extraction was carried out 3 times. All of the filtrates were evaporated in a rotary evaporator under high vacuum to get crude hexane extracts named as CD-M1 0.51 g (hexane extract of C. depressus May, 2016), CD-S2 0.52 g (hexane extract of C. depressus September 2015) and CD-J3 0.50 g (hexane extract of C. depressus January, 2016.
The residual plant material was again soaked in 250 ml methanol. The extraction was carried out 3 times to get crude metanolic extracts named as CD-M4 3.16 g (methanolic extract of C. depressus May, 2016), CD-S5 3.8 g (methanolic extract of C. depressus September 2015) and CDJ6 2.5 g (methanolic extract of C. depressus January, 2016.
Similarly, the residual plant material was again soaked in 250 ml water. The extraction was carried out 3 times. All of the filtrate was evaporated in a rotary evaporator under high vacuum, for complete dryness the temperature was increased to 60°C to get crude water extracts named as CD-M7 1.04 g (methanolic extract of C. depressus May, 2016), CD-S8 0.89 g (methanolic extract of C. depressus September 2015) and CD-J9 0.94 g (methanolic extract of C. depressus January, 2016. Then these hexane, methanolic and water extracts were subjected to α-glucosidase inhibition activity.
The extracts CD-M1 to CD-J9 as given in Table 2 were subjected to α-glucosidase inhibition assay. Acarbose was used as a control with IC50 value 37.38 ± 0.14 μM. The CD-J3, CD-S2 and CD-M1 hexane extracts showed potent remarkable results with IC50 values 8.43 ± 0.12, 26.53 ± 0.15 and 37.28 ±0.16 μg/mL respectively.
| S.No | Photochemical Compounds | Molecular Formula and Molecular Weight (g/mol) | Retention Time |
|---|---|---|---|
| 1. | Decane | C10H22 142.176 |
5.063 |
| 2. | Cyclohexane,1,1-dimethyl | C8H16 112.2126 |
2.770 |
| 3. | Cyclohexane,1,1,3-trimethyl | C9H18 126.2392 |
3.241 |
| 4. | 2,4-dimethyl heptane | C9H20 128.259 |
2.833 |
| 5. | 2,6-dimethyl octane | C10H22 142.282 |
4.353 |
| 6. | Butyl-cyclohexane | C10H20 140.27 |
5.439 |
| 7. | 1-methyl-2- pyrrolidnone | C5H9NO 99.133 |
5.577 |
| 8. | 5-methyl decane | C11H24 156.308 |
5.688 |
| 9. | 2-methyl-tri- decane | C14H30 198.388 |
5.762 |
| 10. | p-Cymene | C10H14 134.222 |
6.053 |
| 11. | Undecane | C11H24 156.313 |
6.164 |
| 12. | Thymol | C10H14O 150.218 |
6.260 |
| 13. | 1,4-dimethyl- 2-ethyl benzene | C10H14 134.218 |
6.291 |
| 14. | Naphthalene, decahydro-2- methyl | C11H20 152.2765 |
6.355 |
| 15. | Azulene | C10H8 128.174 |
7.170 |
| 16. | Tetradecane | C14H30 198.394 |
7.229 |
| 17. | Nonyl-Cyclo Propane | C12H24 168.319 |
9.066 |
| 18. | 5-(1-methyl propyl)- nonane | C13H28 184.224 |
8.928 |
| 19. | 2,6-dimethyl naphthalene | C12H12 156.228 |
9.246 |
| 20. | Dibutyl phthalate | C16H22O4 278.348 |
12.842 |
| 21. | Phthalic acid-di (2-propyl pentyl) ester | C24H38O4 390.556 |
17.952 |
| 22. | Di-iso-octyl phthalate | C24H38O4 390.556 |
17.353 |
Table 1: GC-MS analysis of fraction CDB.
| Sr No. | Code | Inhibition (%) at 0.5 mg/ml | IC50 (μg/ml) |
|---|---|---|---|
| 1. | CD-M1 | 92.28 ± 0.21 | 37.28 ± 0.16 |
| 2. | CD-S2 | 92.46 ± 0.19 | 26.53 ± 0.15 |
| 3. | CD-J3 | 95.72 ± 0.17 | 8.43 ± 0.12 |
| 4. | CD-M4 | 86.45 ± 0.23 | 132.35 ± 0.19 |
| 5. | CD-S5 | 38.25 ± 0.19 | - |
| 6. | CD-J6 | 84.36 ± 0.28 | 145.62 ± 0.21 |
| 7. | CD-M7 | 89.46 ± 0.25 | 82.67 ± 0.17 |
| 8. | CD-S8 | 34.26 ± 0.24 | - |
| 9. | CD-J9 | 89.39 ± 0.21 | 82.54 ± 0.15 |
| 10. | Acarbose | 92.63 ± 0.17 mM | 37.38 ± 0.14 μM |
Table 2: Effect of seasonal variations on α-glucosidase inhibitory activity of C. depressus.
In vitro anti-urease inhibition activity: The 20 mL urease enzyme and 6 mL of phosphate buffer was added in it. Both the contents were mixed up properly and placed in the wells of plates, preincubated for 10 minutes at 25°C and test compound 5 mL was added in it. This solution was again incubated for 10 minutes at 25°C and 20 mM urea 15 mL was added. This mixture was again incubated for 10 minutes at 25°C and RGI 2 (100 ml) was added in it and incubated for 25 minutes at room temperature. Gen 5 software was used on ELISA reader for measuring the absorbance at 630 nm. EZ-Fit enzyme kinetics software (Perrella Scientific Inc.,
Amherst, MA., USA) was used for calculating the IC50 values of the fractions. The percentage of inhibition was calculated by using the following formulae [8,9].
The fractions CD-1 to CD-24 were subjected to anti-urease inhibition assay. Thiourea was used as a control. None of the fraction showed potent IC50 values. CD1 was the pure compound named dioctyl phthalate.
‘This study suggests that is prudent to adjust the screen brightness than needed to do the work or enjoy the entertainment.
In vivo α-glucosidase activity of Corchorus depressus Linn: Both sexes of Albino Sprague-Dawley rats that weighed 200-250 gm of body weight were selected and kept in the animal room of Pharmacology Research Laboratory at the Faculty of Pharmacy and Alternative Medicines, the Islamia University of Bahawalpur. The animals were housed in polycarbonated cages of size 47 × 34 × 18 cm3 with maximum of three animals per cage. The standard conditions of temperature and humidity that were maintained throughout the study were (25 ± 2°C) and (55-55%) along with exposure to a 12:12 hours light and dark cycle,throughout. Animals had been supplied with a normal animal diet and allowed to drink water ad libitum. Animals had been familiarized with the experimental conditions for one week before the start of study to minimize animal stress. The study protocols and procedures had been approved by the ethics committee of animal handling of the department of pharmacy, the Islamia University of Bahawalpur.
Induction of diabetes mellitus: Diabetes mellitus had been induced pharmacologically using Alloxan monohydrate. Alloxan selectively inhibited glucose induced insulin secretion by inhibiting the glucokinase and caused a state of insulin dependent diabetes mellitus (type-1) through its ability to induce a selective necrosis of the β cells of pancreas [10]. Alloxan can be administered intravenously (65 mg/kg) but an intraperitoneal dose below 150 mg/kg may be insufficient for the induction diabetes in rats.
Drug administration: After application of alloxan blood glucose at fifth day of initial dose committed the presence of disease (DM), animals were allowed to stabilize for one week. On the day seventh, animals were divided into different groups and their fasting blood glucose levels was determined. The treatment was started on the same day and was considered the day one of the study Lasker K, et al. [11] and Lenzen S [12]. Furthermore estimation of fasting blood glucose level was carried out on 1st and 4th days of the study as given.
Assay for determination of blood glucose level: Blood glucose level was determined using a single touch glucometer (Accu- chek Performa, Roche, Germany) with test strips capable of measuring blood glucose level in the range of 10 mg/dL to 600 mg/dL. Test strips used for blood glucose estimation contains test area that is incorporated within chemicals which are highly sensitive. When blood comes in contact with this sensitive area, a chemical reaction starts that is designated as glucose dye oxidoreductase mediator reaction (PQQ-dependent glucose dehydrogenase mediator reaction). The consequence of this reaction is change in color of test strip area. The meter is designed to detect this change quantitatively and convert this change into blood glucose level.
Calculations and statistical examination
The percentage inhibition was calculated by the following equation.
Inhibition (%)=(Abs. of Control–Abs. of Test/Abs. of Control 100
The fractions CD-1 to CD-14 were subjected to α-glucosidase inhibition assay and their IC50 values were determined by using EZ-Fit enzyme kinetics (Perrella Scientific Inc, Amherst, USA) software. Test solutions were assayed at various dilutions e.g. 0.5, 0.25, 0.125 and 0.0625 mg/ml. All the results were mean ± SEM and n=5. Student t test was used. P values are considered as P < 0.05=significant (*), P < 0.01=more significant (**) and P < 0.001=highly significant (***). All extract treated groups and standard controlled were compared to positive control and positive control was compared to normal control.
Phytochemical investigation
Phytochemical investigation was carried out with the help of GCMS and LC-MS analysis.
The fraction CDB (10% EtOAc/Pet. ether) was subjected to GCMS and 22 metabolites were isolated.
Based on LCMS analysis and α-glucosidase inhibitory profile, it is augmented that betulinic acid, canarigenin and strophanthidin might be the potent biological markers for anti-diabetic activity. Detailed studies needed to be done to further confirm this assumption. The structures cannot be assigned to the rest of the peaks.
For the confirmation of traditional antidiabetic use, the fractions CD-2 to CD-24 of crude methanolic extract of Corchorusdepressuswere tested for in vitro α-glucosidase activity. The fractions CD-14 CDB (10% EtOAc/Pet. Ether), CD-2 CDC (10% EtOAc/Pet. Ether), CD-3 CDD (10% EtOAc/Pet. Ether), CD-4 CDE (10% EtOAc/Pet. Ether), CD-5 CDF (20% EtOAc/ Pet. Ether), CD-7 CDG (30% EtOAc/Pet. Ether), CD-8 CDH (40% EtOAc/Pet. Ether), CD-9 CDI (50% EtOAc/Pet. Ether), CD- 10 CDJ (50% EtOAc/Pet. Ether), CD-12 CDK (50% EtOAc/Pet. Ether), CD-13 CDA obtained by eluting column at 16% EtOAc/ Pet Ether, CD-15 CDL (70% EtOAc/Pet. Ether), CD-16 CDM (90% EtOAc/Pet. Ether), CD-17 CDN (100% EtOAc), CD-18 CDO (10% MeOH/EtOAc), CD-19 CDQ (20% MeOH/EtOAc), CD-24 CDR (100% MeOH) respectively. The fractions CD-2, CD- 3, CD-5, CD-6, CD-7, CD-13, CD-14, CD-15, CD-16, CD-17 and CD-20 showed inhibition against α-glucosidasewith IC50 values 13.16 ± 0.09, 31.54 ± 0.05, 45.32 ± 0.12, 12.75 ± 0.07, 46.24 ± 0.09, 38.12 ± 0.06, 4.31 ± 0.07, 6.32 ± 0.08, 15.37 ± 0.13, 23.75 ± 0.1, 12.42 ± 0.13 and 27.65 ± 0.17 μg/mL respectively as compared to acarbose with IC50 37.38 ± 0.12 μM.
The fractions CD-2 to CD-24 of crude methanolic extract of Corchorus depressus were tested for in vitro anti-urease activity and their IC50 values were determined. Almost all the fractions found to be inactive for anti-urease assay as given in Table 3. Thiourea was used as a control. None of the fraction showed potent IC50 values. CD-1 was the pure compound named dioctyl phthalate.
| S.No | Sample Codes of Fractions | Inhibition (%) at 0.25 mg/mL | IC50 μg/mL |
|---|---|---|---|
| 1. | CD-1 Pure Compound | 21.9 ± 0.3 | Nill |
| 2. | CD-2 | 16.7 ± 0.1 | Nill |
| 3. | CD-3 | 22.1 ± 0.5 | Nill |
| 4. | CD-4 | 34.8 ± 0.3 | Nill |
| 5. | CD-5 | 31.5 ± 0.6 | Nill |
| 6. | CD-6 | 28.3 ± 0.5 | Nill |
| 7. | CD-7 | 14.5 ± 0.2 | Nill |
| 8. | CD-8 | 6.4 ± 0.1 | Nill |
| 9. | CD-9 | 17.6 ± 0.4 | Nill |
| 10. | CD-10 | 11.4 ± 0.2 | Nill |
| 11. | CD-11 | 23.8 ± 0.1 | Nill |
| 12. | CD-12 | 11.6 ± 0.3 | Nill |
| 13. | CD-13 | 8.4 ± 0.1 | Nill |
| 14. | CD-14 | 7.9 ± 0.2 | Nill |
| 15. | CD-15 | 51.36 ± 0.17 | < 250 |
| 16. | CD-16 | 51.24 ± 0.16 | < 250 |
| 17. | CD-17 | 12.34 ± 0.11 | Nil |
| 18. | CD-18 | 52.16 ± 0.18 | < 250 |
| 19. | CD-19 | 25.86 ± 0.18 | Nill |
| 20. | CD-24 | 22.84 ± 0.13 | Nill |
| 21. | Thiourea | 98.21 ± 0.18 | 21.5 ± 0.15 |
Table 3: Anti-urease inhibition of various fractions of methanolic extract of Corchorus depressus Linn.
The crude methanolic extract of Corchorus depressus was also tested for in vivo anti-diabetic activity (Table 4). The crude methanolic extract of plant was dissolved in normal saline and was inserted intraperitoneally in alloxan induced diabetic rats at doses of 50, 75 and 100 mg/kg. At the beginning, blood glucose level was 104 ± 2.9 mg/dL for normal control group, 476.7 ± 4.0 mg/dL was the blood glucose value of positive control group and 478 ± 6.2 mg/ dL was the glucose level of standard control group. Glucose level of crude methanolic extract of C. depressus at 50, 75 and 100 mg/ kg group was observed 467.5 ± 14.5, 469.5 ± 2.5, 479.5 ± 5.2 mg/ dL, respectively.
| S.No | Sample Codes of Fractions |
Inhibition (%) at 0.5 mg/mL |
IC50 (μg/mL) |
Peak Area |
|---|---|---|---|---|
| 1. | CD-2 | 96.12 ± 0.13 | 13.16 ± 0.09 | 1.15 |
| 2. | CD-3 | 94.12 ± 0.15 | 31.54 ± 0.05 | 0.96 |
| 3. | CD-4 | 92.19 ± 0.17 | 45.32 ± 0.12 | 0.95 |
| 4. | CD-5 | 96.51 ± 0.13 | 12.75 ± 0.07 | 0.70 |
| 5. | CD-6 | 94.21 ± 0.14 | 46.24 ± 0.09 | 0.24 |
| 6. | CD-7 | 93.17 ± 0.12 | 38.12 ± 0.06 | 0.43 |
| 7. | CD-8 | 48.31 ± 0.15 | - | 0.46 |
| 8. | CD-9 | 23.52 ± 0.16 | - | 0.15 |
| 9. | CD-10 | 12.47 ± 0.18 | - | 0.21 |
| 10. | CD-11 | 13.76 ± 0.17 | - | 0.34 |
| 11. | CD-12 | 74.19 ± 0.16 | 173.12 ± 0.12 | 0.85 |
| 12. | CD-13 | 97.25 ± 0.14 | 4.31 ± 0.07 | 0.17 |
| 13. | CD-14 | 96.72 ± 0.18 | 6.32 ± 0.08 | 0.13 |
| 14. | CD-15 | 93.52 ± 0.16 | 15.37 ± 0.13 | 0.24 |
| 15. | CD-16 | 93.42 ± 0.22 | 23.75±0.16 | 0.49 |
| 16. | CD-17 | 94.68 ± 0.18 | 12.42 ± 0.13 | 0.56 |
| 17. | CD-18 | 95.46 ± 0.19 | 16.52 ± 0.14 | 0.26 |
| 18. | CD-19 | 34.75 ± 0.15 | - | 0.11 |
| 19. | CD-20 | 92.48 ± 0.23 | 27.65 ± 0.17 | 0.04 |
| 20. | CD-21 | 81.32 ± 0.27 | 217.27 ± 0.23 | 0.08 |
| 21. | CD-22 | 91.57 ± 0.22 | 62.54 ± 0.16 | 4.39 |
| 22. | CD-23 | 83.15 ± 0.29 | 172.23 ± 0.21 | 0.00 |
Table 4: α-Glucosidase inhibition of various fractions of methanolic extract of Corchorusdepressus Linn.
On the first day, 107.1 ± 2.1 mg/dL was glucose level of normal control group, 485.9 ± 3.1 mg/dL and 90.8 ± 6.6 mg/dL was the value of observed blood glucose of standard control group, 290.0 ± 20.0 mg/dL was reduced value of blood glucose for crude extract of C. depressus 50 mg/kg group, 149.0 ± 4.0 mg/dL was observed value for 75 mg/kg and 115.8 ± 6.4 mg/dL was observed blood glucose value for 100 mg/kg group. On fourth day, 109 ± 1.3 mg/dL was observed value for standard control group, 106.5 ± 4.1 mg/dL was measured value for standard control group, 208.5 ± 3.5 mg/dL was the calculated amount of blood glucose for C. depressus 50 mg/kg whereas 129.5 ± 7.5 mg/dL was blood glucose level observed for 75 mg/kg and 106.0 ± 2.2 mg/dL was blood glucose level observed for 100 mg/kg of concentration. Results of crude extracts of C. depressus at 100 mg/kg were more significant as compared to 75 and 50 mg/kg. It was observed that the dose dependent anti-diabetic activity portrait of C. depressus was observed to be similar to the standard drug, glibenclamide.
Table 5 showed the values with reduction in blood glucose level. In this profile, alloxan was used to induced diabetes mellits. Alloxan inhibited the glucokinase and inhibits glucose, a state of insulin dependent diabetes mellitus (IDDM) by discerning necrosis of β-cells of pancreas [13-20].
| S.No | Treatment Groups | Glucose Level (mg/dL) | ||
|---|---|---|---|---|
| 0 Day | 1st Day | 2nd Day | ||
| 1. | Normal Control (Citrate Buffer, 4ml/kg) | 104.1 ± 2.9 | 107.1 ± 2.1 | 109.1 ±1.3 |
| 2. | Positive Control (N/S, 4ml/kg) |
476.7 ± 4.0 | 485.9 ± 3.1 | 468.3 ± 2.7 |
| 3. | Standard Control (Glibenclamide 10mg/kg) |
478.0 ± 6.2 | 90.83 ± 6.6*** | 106.5 ± 4.1*** |
| 4. | Cd.Cr (50mg/kg) | 467.5 ± 14.5 | 290.0 ± 20.0*** | 208.5 ± 3.5*** |
| 5. | Cd.Cr (75mg/kg) | 469.5 ± 2.5 | 149.0 ± 4.0*** | 129.5 ± 7.5*** |
| 6. | Cd.Cr (100mg/kg) | 479.6 ± 5.2 | 115.8 ± 6.4*** | 106.0 ± 2.2*** |
Table 5: Effects of intra-peritoneal administration of different doses of C. depressus Linn. Inalloxan induced diabetic rats for 4 days.
The fraction CDB (10% EtOAc/Pet. ether) showed remarkable α-glucosidase activity with IC50 6.32 ± 0.08 μg/mL. So it was sent for GC-MS analysis. The GC-MS analysis of sub-fraction CDB (0.054 g) was done and 22 compounds were isolated which belongs to hydrocarbons, heterocyclics, monocyclic terpenoids and phthalate class of compounds.
Moreover LCMS analysis of fractions CD-23, CD-22, CD-21, CD- 20 and CD-13 was done. The fraction CD-23 (10% EtOAc/Pet. ether) showed the presence of strophanthidin, fraction CD-21 CDE (10% EtOAc in Pet. ether) also resulted in strophanthidin. However, CD-22 CDF (20% EtOAc in Pet. Ether) the structure of not any compound was determined from this fraction, CD-20 CDG (30% EtOAc/Pet. ether) results in the isolation of iso-rhamnetin, strophanthidin, β-sitosterol and fraction CD-13 (16% EtOAc/Pet. ether) resulted in canarigenin and betulinic acid, respectively. The structures cannot be assigned to the rest of the peaks.
Based on LCMS analysis and α-glucosidase inhibitory profile, it is augmented that betulinic acid, canarigenin and strophanthidin might be the potent biological markers for anti-diabetic activity. Detailed studies needed to be done to further confirm this assumption (Figure 2) ( Table 6-10) [20-24].
Figure 2: Anti-diabetic activity of Corchorus depressus.
Based on experimental data, it was concluded that crude extract of C. depressus has good anti-diabetic potential. The methanolic extract and its various fractions in pet ether and ethyl-acetate showed remarkable inhibitory activities against α-glucosidase. Fractions CD-2, CD-3, CD-4, CD-5, CD-6, CD-7, CD-13, CD-14, CD-15, CD-16, CD-17 and CD-20 showed inhibition against α-glucosidase with IC50 values 13.16 ± 0.09, 31.54 ± 0.05, 45.32 ± 0.12, 12.75 ± 0.07, 46.24 ± 0.09, 38.12 ± 0.06, 4.31 ± 0.07, 6.32 ± 0.08, 15.37 ± 0.13, 23.75 ± 0.1, 12.42 ± 0.13 and 27.65 ± 0.17μg/mL, respectively, as compared to acarbose with IC50 37.38 ± 0.12 μM.
The GC-MS analysis of CD-14 CDB results in the isolation of phytochemicals as given. The LC-MS analysis of the fractions CD- 23, CD-22, CD-21, CD-20 and CD-13 gave rise to strophanthidin, iso-rhamnetin, β-sitosterol, canarigenin and betulinic acid respectively. Strophanthidin was observed in CD-23, CD-21 and CD-20 respectively. So it can be served as potent marker for α-glucosidase inhibitor. None of the phytochemical was similar in GC-MS and LC-MS analysis. But these non-polar fractions were inactive for anti-urease activity.
| S.No | Compound Name | Compound Structure | Molecular Formula | Molecular Weight (g/mol) | Scan Time (min.) |
|---|---|---|---|---|---|
| 1. | Not determined | _ | _ | 413 | 1.04 |
| 2. | _ | _ | _ | 429 | 1.6 |
| 3. | _ | _ | _ | 428 | 2.23 |
| 4. | _ | _ | _ | 803 | 3.6 |
| 5. | Strophanthidin | 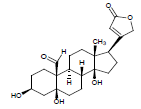 |
C23H32O6 | 405 | 4.1 |
| 6. | _ | _ | _ | 609 | 4.2 |
| 7. | _ | _ | _ | 463 | 4.4 |
| 8. | _ | _ | _ | 785 | 4.6 |
| 9. | _ | _ | _ | 901 | 4.8 |
| 10. | _ | _ | _ | 960 | 4.94 |
| 11. | _ | _ | _ | 615 | 5.04 |
| 12. | _ | _ | _ | 795 | 5.44 |
| 13. | _ | _ | _ | 919 | 5.6 |
| 14. | _ | _ | _ | 953 | 5.75 |
| 15. | _ | _ | _ | 838 | 6.41 |
| 16. | _ | _ | _ | 921 | 7.1 |
| 17. | _ | _ | _ | 955 | 7.33 |
| 18. | _ | _ | _ | 981 | 7.74 |
| 19. | _ | _ | _ | 981 | 8.1 |
| 20. | _ | _ | _ | 969 | 7.93 |
| 21. | _ | _ | _ | 887 | 7.3 |
| 22. | _ | _ | _ | 982 | 7.1 |
| 23. | _ | _ | _ | 620 | 5.1 |
| 24. | _ | _ | _ | 803 | 4.84 |
| 25. | _ | _ | _ | 427 | 1.6 |
Table 6: LC-MS analysis of fraction CD-23.
| S.No | Compound Name | Compound Structure | Molecular Formula | Molecular Weight (g/mol) | Scan Time (min.) |
|---|---|---|---|---|---|
| 1. | _ | _ | _ | 144 | 0.67 |
| 2. | _ | _ | _ | 413 | 3.10 |
| 3. | _ | _ | _ | 803 | 4.06 |
| 4. | _ | _ | _ | 609 | 4.17 |
| 5. | _ | _ | _ | 945 | 4.4 |
| 6. | _ | _ | _ | 785 | 4.6 |
| 7. | _ | _ | _ | 901 | 4.8 |
| 8. | _ | _ | _ | 959 | 4.93 |
| 9. | _ | _ | _ | 723 | 5.7 |
| 10. | _ | _ | _ | 898 | 6.2 |
| 11. | _ | _ | _ | 956 | 6.34 |
| 12. | _ | _ | _ | 981 | 6.9 |
| 13. | _ | _ | _ | 831 | 7.10 |
| 14. | _ | _ | _ | 931 | 8.1 |
| 15. | _ | _ | _ | 947 | 6.61 |
| 16. | _ | _ | _ | 804 | 4.8 |
| 17. | _ | _ | _ | 954 | 8.1 |
| 18. | _ | _ | _ | 947 | 6.35 |
Table 7: LC-MS analysis of fraction CD-23.
| S.No | Compound Name | Compound Structure | Molecular Formula | Molecular Weight (g/mol) | Scan Time (min.) |
|---|---|---|---|---|---|
| 1. | _ | _ | _ | 284 | 1.43 |
| 2. | _ | _ | _ | 349 | 1.6 |
| 3. | _ | _ | _ | 284 | 1.85 |
| 4. | _ | _ | _ | 217 | 2.5 |
| 5. | _ | _ | _ | 301 | 2.7 |
| 6. | _ | _ | _ | 259 | 2.8 |
| 7. | _ | _ | _ | 567 | 3.7 |
| 8. | _ | _ | _ | 361 | 3.10 |
| 9. | Strophanthidin | 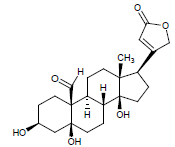 |
C23H32O6 | 405 | 4.04 |
| 10. | _ | _ | _ | 945 | 4.4 |
| 11. | _ | _ | _ | 785 | 4.6 |
| 12. | _ | _ | _ | 901 | 4.9 |
| 13. | _ | _ | _ | 902 | 4.98 |
| 14. | _ | _ | _ | 960 | 5.03 |
| 15. | _ | _ | _ | 703 | 5.3 |
| 16. | _ | _ | _ | 709 | 5.45 |
| 17. | _ | _ | _ | 870 | 5.62 |
| 18. | _ | _ | _ | 986 | 5.95 |
| 19. | _ | _ | _ | 956 | 6.33 |
| 20. | _ | _ | _ | 910 | 6.42 |
| 21. | _ | _ | _ | 831 | 7.1 |
Table 8: LC-MS results of fraction CD-21.
| S.No | Compound Name | Compound Structure | Molecular Formula | Molecular Weight (g/mol) | Scan Time (min.) |
|---|---|---|---|---|---|
| 1. | _ | _ | _ | 118 | 0.52 |
| 2. | _ | _ | _ | 217 | 2.5 |
| 3. | iso-rhamnetin | 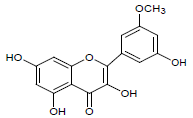 |
C16H12O7 | 317 | 3.10 |
| 4. | Strophanthidin | 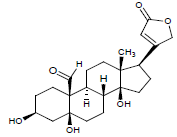 |
C23H32O6 | 405 | 4.1 |
| 5. | β-Sitosterol | 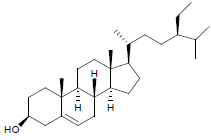 |
C29H50O | 415 | 4.2 |
| 6. | _ | _ | _ | 518 | 4.4 |
| 7. | _ | _ | _ | 785 | 4.7 |
| 8. | _ | _ | _ | 902 | 4.9 |
| 9. | _ | _ | _ | 690 | 4.10 |
| 10. | _ | _ | _ | 615 | 5.04 |
| 11. | _ | _ | _ | 497 | 5.2 |
| 12. | _ | _ | _ | 703 | 5.31 |
| 13. | _ | _ | _ | 927 | 5.8 |
| 14. | _ | _ | _ | 831 | 7.07 |
| 15. | _ | _ | _ | 831 | 7.23 |
Table 9: LC-MS results of fraction CD-20.
| S.No | Compound Name | Compound Structure | Molecular Formula | Molecular Weight (g/mol) | Scan Time (min.) |
|---|---|---|---|---|---|
| 1. | _ | _ | _ | 322 | 1.13 |
| 2. | Canarigenin | 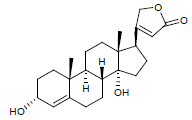 |
C23H32O4 | 373 | 1.7 |
| 3. | Betulinic Acid | 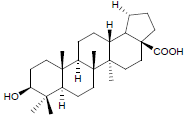 |
C27H44O3 | 417 | 2.22 |
| 4. | _ | _ | _ | 452 | 3.23 |
| 5. | _ | _ | _ | 671 | 4.14 |
| 6. | _ | _ | _ | 733 | 4.90 |
| 7. | _ | _ | _ | 795 | 5.43 |
| 8. | _ | _ | _ | 851 | 5.71 |
| 9. | _ | _ | _ | 487 | 5.70 |
| 10. | _ | _ | _ | 980 | 6.1 |
| 11. | _ | _ | _ | 981 | 6.4 |
| 12. | _ | _ | _ | 831 | 7.06 |
| 13. | _ | _ | _ | 835 | 7.21 |
Table 10: LCMS results of fraction CD-13.
The CD-J3, CD-S2 and CD-M1 hexane extracts showed potent remarkable results with IC50 values 8.43 ± 0.12, 26.53 ± 0.15 and 37.28 ± 0.16 μg/mL respectively.
The extracts CD-M7 and CD-J9, May and January water extracts of C. depressus showed best anti-urease activities with IC50 1.63 ± 0.08 and 2.42 ± 0.07 μg/mL, as compared to thiourea with IC50 21.46 ± 0.13 μM respectively.
So it was concluded from the above discussion that non-polar CD fractions (upto 20% EtOAc/Pet. ether) showed potent IC50 values for α-glucosidase inhibition but CD polar (water extracts) showed remarkable IC50 values for anti-urease inhibition.
Dioctyl phthalate 791.6 mg from fraction CDA and CDF toxicity was checked on animal model. Rats were dyeing on high dose 100 mg/kg of phthalate injection. DOP was confirmed by co TLC by using analytical grade solvent is little toxic to this plant. If we remove phthalate from this plant, it will be more effective for curing diabetes.
Citation: Latif I, Latiff R, Khan I, Mushtaq MM, Zubair M, Ain Q, et al. (2021) Qualitative Studies on Anti-diabetic Activity of Corchorus depressus. J Appl Pharm. 13:323.
Received: 08-Nov-2021 Accepted: 22-Nov-2021 Published: 29-Nov-2021
Copyright: © 2021 Latif I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.