Journal of Molecular Imaging & Dynamics
Open Access
ISSN: 2155-9937
ISSN: 2155-9937
Research Article - (2012) Volume 2, Issue 1
Dynamic positron emission tomography (PET) imaging of with L-[methyl- 11 C]methionine (11C-MET) was developed in the late 1990’s to non-invasively estimate skeletal muscle protein synthesis, but no studies have shown that the measurements respond to resistance exercise, which stimulates protein synthesis in humans. Ten healthy women aged 25-75 years underwent a 14-hour fast, followed by unilateral knee extension and flexion exercise and consumption of an 8-ounce serving of fruit juice. Five subjects underwent dynamic 11C-MET PET imaging of the mid-thigh 2-3 hours after exercise and five were imaged 1 hour after exercise. Images were processed to obtain the Patlak slope K i , which describes the fractional extraction rate of 11C-MET into skeletal muscle protein. Additionally, the images were processed with a three-compartment kinetic model to determine rate constants for 11C-MET transport between muscle tissue, protein and plasma. All subjects showed excellent mid-thigh uptake of 11C-MET. Subjects imaged 2-3 hours after exercise showed no unilateral enhancement. However, subjects imaged one hour post-exercise showed an enhancement of 11C-MET uptake in the exercised leg compared to the control leg, corresponding to K i elevations between 3.8% - 31.1%. From the three-compartment analysis, the increased uptake corresponded primarily to an increased rate constant for extraction of 11C-MET from plasma to skeletal muscle tissue. Finally, older subjects tended to have smaller values of K i than the younger subjects. In summary, 11C-MET kinetics is responsive to a unilateral exercise stimulus, and this technique may prove useful to study skeletal muscle amino acid kinetics in response to exercise, aging and other conditions
Keywords: Positron emission tomography, Radioisotopes, Scanning, Therapeutic response, Skeletal Muscle, Quantification
Aging is characterized by loss of skeletal muscle mass and an even larger deterioration of muscle strength [1]. These changes are associated with impaired performance and mobility, and increased risk of falls and injuries, among the growing elderly population [2-5]. Age-related muscle atrophy results in good part from disruption in the normal function of skeletal muscle tissue, which involves a dynamic balance between synthesis of muscle protein from amino acids in the cellular milieu and dissociation of protein into free amino acids [6]. The alterations in skeletal muscle amino acid metabolism that occur with age are complex, but generally result from suppression of factors that promote protein synthesis, such as IGF-1, and enhancement of other factors, such as inflammatory cytokines and cortisol, that enhance protein degradation. Protein synthesis is particularly affected by age. Sarcopenia, a condition that represents the extreme of age-related skeletal muscle atrophy, is associated with a roughly 30% decline in myosin heavy chain synthesis [7]. Loss of capacity to synthesize skeletal muscle protein not only results in loss of muscle mass and function, but also impairs the ability of skeletal muscle to repair damage and to respond to interventions such as exercise. Thus, measurements of protein synthesis rates are an important tool for investigating the effect of aging on skeletal muscle, and for understanding variations in the anabolic response of muscle tissue to exercise and other interventions.
Clinical research studies of age-related muscle loss and anabolic response to interventions often employ clinical imaging technologies to track the morphology and composition of skeletal muscle tissue as etiologic factors in disability and as characteristics that respond to therapy. X-ray computed tomography (CT), magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) have been employed in the clinical research setting to estimate physical properties such as muscle cross-sectional area [3,4] and fatty infiltration of skeletal muscle [1,4,8]. Positron emission tomography (PET) and MRS have also been employed to assess skeletal muscle energetics, but to date, only one study has used imaging to characterize skeletal muscle amino acid transport and protein synthesis, a metabolic characteristic which is closely tied to skeletal muscle atrophy and to the anabolic response of the tissue to exercise and other interventions. In 1998, Fischman et al. [9] published a new technique for the measurement of muscle protein synthesis in using PET with L-[methyl-11C]methionine (11C-MET). Previous research in their laboratory using anesthetized dogs, led to the discovery that protein synthesis rate (PSR) evaluated in the paraspinous muscle using PET was highly correlated to measurements obtained from necropsy of that muscle following infusion of the 13C-labelled analog [10]. Another study in rats showed that 11C-MET was negligibly influenced by trans-methylation, an effect which causes the radiolabel to be released to the blood, confounding the PET measurement. The clinical study by Fischman et al. [9] showed 11C-MET had excellent uptake in the muscles of adult humans, and determined the variation of skeletal muscle protein synthesis rates estimates in the mid-thigh.
After this initial work, 11C-MET imaging of skeletal muscle was never employed systematically to study skeletal muscle in relation to age-related changes and to interventions. However, since the time of these publications in the late 1990’s, there has been considerable evolution in PET technology, including the widespread installation of cyclotrons at major hospital facilities and the growth of a large installed base of modern volumetric positron emission tomography/computed tomography (PET/CT) systems. The more widespread uptake permits a broader application of this approach in clinical research studies, and the development of volumetric PET/CT permits a combined threedimensional assessment of anatomy and function in multiple muscle beds. In this study, we report results of a pilot investigation of 11C-MET PET imaging with a modern volumetric PET/CT scanner. The goals of our study were to reproduce the imaging findings of Fischmann et al. [9], to determine if incorporation of 11C-MET into skeletal muscle showed an expected acute response to resistance exercise, and in order to set the stage for larger scale studies, to obtain kinetic data in both young and elderly subjects.
Subjects
Because elderly females are strongly affected by sarcopenia, our study population comprised only women. We included healthy females aged 25-35 years or aged 70 years or greater who had not engaged in an structured exercise program within the previous six months. We recruited 10 healthy adult female volunteers to participate in the study. Seven of the subjects were young women between 25 and 35 years of age and 3 subjects were elderly women more than 70 years old. The study was approved by the University of California, San Francisco Committee for Human Research, and written informed consent was obtained from each subject prior to commencement of study procedures.
Exercise sessions
Subjects reported to our exercise laboratory on two separate occasions, 2-4 days apart. The first visit served as a familiarization session and as a means of determining the subjects’ approximate 10-repetition maximum (10RM) weight for knee extension and knee flexion. For all subjects the right leg was exercised and the left was used as a non-exercise control. Briefly, a weight was selected randomly and the subject was asked to attempt 10 repetitions. If the subject could easily complete 10 repetitions, weight was increased and the process repeated after 3-5 minutes of rest. The weight that caused the subject to reach failure at or near the 10th repetition was recorded and used as the weight lifted for the exercise trial on the day of the PET/CT imaging session. Subjects were asked to limit physical activity to “necessary” activities of daily living (i.e. no structured exercise) for 48 hours before the scheduled exercise and imaging session. On the day of the imaging session subjects completed 3 sets of 10 repetitions of single leg knee extension and flexion using the weight that was obtained during the familiarization session as described above. Immediately after the exercise session, subjects were given 8 fluid ounces (~235 ml) of fruit juice. Then, subjects were escorted to the imaging suite where they rested while seated until the commencement of the PET/CT imaging procedures (approximately 1-3 hours).
Radiosynthesis of 11C-MET
11C-MET was prepared by the method described by Pascali et al. [11] with minor modifications. Radiochemical purity of the final product was routinely >99%.
PET/CT Imaging
All images were acquired using a Discovery VCT PET/CT camera (GE Healthcare, St. Chalfont, UK). The subject was oriented in the scanner in the supine position, with legs extended into the scanner aperture, with feet straight up and taped together. An anteroposterior scout view was used to depict the lower body anatomy from the iliac crest to the lower knee. The scout view was employed to determine a CT scanning interval from the mid thigh to a point roughly 2 cm superior to the distal femoral condyle. The dynamic PET axial field of view, which extended 14.5 cm along the table axis (the fixed width of the PET detector array), was centered in the CT volume. Based on the CT scan volume defined from the scout view, the subject was imaged with a helical CT scan (120 kVp, 300 mAs, 3.75 mm sections, 5122 matrix, standard reconstruction kernel). After the CT image was acquired, the subject was injected with a bolus of 592-999 MBq 11C-MET solution, resulting in an approximately 4 mSv effective absorbed dose to the subjects. Beginning concurrently with the 11C-MET bolus injection, the dynamic PET data were acquired using a sequence of 27 temporally contiguous frames over the course of 60 min. The time frames were of variable durations, acquired in the following sequence: [15 x 10 sec., 5 x 30 sec., 4 x 5 min., 2 x 10 min., 1 x 15 min.]. Images were reconstructed into voxels of dimensions 1.95 x 1.95 x 3.27 mm3 in 256 x 256 x 47 matrices via a 3D ordered subset expectation maximization algorithm provided by the scanner manufacturer. Figure 1 shows a 3D rendering of a volumetric PET image from one of our study subjects obtained by summing the data from all of the frames.
Derivation of the arterial input function
To estimate the contribution of arterial blood flow to the skeletal muscle uptake of 11C-MET as a function of time, we derived the arterial input function Cp(t) from region of interest analysis around the femoral artery as described by Croteau et al. [12]. The input function was obtained by superposition of regions in the arteries and surrounding tissue, which were combined so as to correct for partial volume errors due to scanner resolution. To define these arterial regions, early time-frames of the dynamic PET image (usually occurring 30-50 sec. after tracer injection) were used to segment the femoral arteries and surrounding tissue. In these frames, the tracer had just been injected, so activity was largely restricted to the intravascular space. Regions in the interior of the arteries were obtained by including only those voxels whose value was greater than 90% of the maximum value in the early frames of the acquisition. In each transaxial slice, horizontal profiles were then taken through the centroids of the segmented arterial crosssections and the full width at half maximum (FWHM) was computed. In each slice, annuli surrounding the arteries were defined using the FWHM, where the annuli had inner radii of 0.5 FWHM and outer radii of 1 FWHM, and were centered on the centroids. The region defined by the initial thresholding step was considered the primary arterial region (ART), and the annular region between the inner and outer radius was considered as a background (BG) region. The spill-in from the background into the inner region was taken as SPI=KSP *BG, where KSP is a spill-in coefficient. The true plasma concentration Cp(t) was estimated as:

Where CART(t) is the concentration in the inner region, and RC is a recovery coefficient which corrects for the “spill-out” of activity from the primary arterial region due to partial volume averaging. Figure 2 shows a typical input function derived using this methodology compared to input function data from an arterial line obtained in the same subject.
Figure 2: (a) Typical arterial input function from analysis of femoral artery from dynamic images. (b) Image from early frame of acquisition showing artery and annular region used for background subtraction (c) Plot of arterial input function (circles) superimposed on plot of image-derived input function (dashed line).
Derivation of tissue activity curves
In order to quantify the uptake of 11C-MET in skeletal muscle tissue as a function of time, tissue activity curves (TAC) were generated from regions of interest (ROIs) determined automatically from the CT scans using threshold-driven region growing. The distal femoral condyle and the lesser trochanter, two bony landmarks visible on the anteroposterior scout view, were used to establish the inferior and superior limits of the set of axial slices included in the TAC ROI. The inferior limit of the ROI occurred in the axial slice closest to 36% of the distance from the femoral condyle to the lesser trochanter, and the superior limit occurred in the slice closest to 44% of that distance. To avoid contamination of the tissue region by arterial counts, a region surrounding the artery, roughly the extent of the outer radius of the annular region used to subtract tissue background counts from the arterial signal, was excluded from the skeletal muscle tissue ROI. Figure 3 shows the TAC derived from regions of interest placed on three typical transverse sections from one of the subjects.
Figure 3: (a-top row) Selected CT axial cross sections through the mid thigh (a-middle row) muscle boundaries derived from segmentation, showing exclusion regions for the femur and the femoral arteries (a-bottom row) muscle boundaries transposed from CT image to axial cross sections of corresponding PET image. (b) Anteroposterior scoutview showing definition of superior and inferior extents of analytic region of interest to define TAC. (c) Cross-section through PET image showing muscle region used for TAC generation and (d) plot of left (lower curve) and right leg (upper curve) TACs.
Three-compartment kinetic model
Image data were analyzed according to a three compartment model as described previously [9] but briefly summarized here. Figure 4 illustrates the model. In this model, the tracer can be found in one of three states: (i) as free methionine in blood plasma, (ii) as free methionine in tissue; or (iii) as methionine bound in protein. The rate constants K1, k2, and k3, describe the exchange of 11C MET between the model’s compartments. K1 describes clearance of tracer from the blood plasma into tissue, k2 describes the exchange of tracer from tissue back into the plasma, and k3 describes the rate at which free tracer in tissue becomes bound in protein. This model assumes that once tracer becomes bound in protein, it does not change state in the timeframe of the study. Based on mass balance, the change in concentration of 11C-MET in the muscle tissue compartment (CT(t)) can be written as:
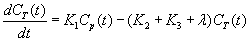
where is the radioactive decay constant for 11C. Assuming that the dissociation of 11C-MET from the bound protein compartment is negligible within the timescale of the imaging study, the change in concentration in the bound skeletal muscle protein compartment CBPR(t) can be written as:
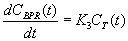
Because the PET scanner cannot spatially resolve the partition of 11C-MET between the tissue and bound protein compartments of the skeletal muscle, the tissue activity curve, which is directly measured, is written as:
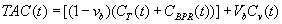
Where vb is the vascular fraction in the tissue compartment and Cv(t) is the concentration of 11C-MET in the vascular fraction. Finally, to obtain the model parameters K1, K2, K3 and vb, the tissue activity curve TAC (t) was fitted to the plasma input function Cp (t) using least squares fitting (Marquardt algorithm, PKIN, PMOD Technologies, Zurich, Switzerland).
Patlak graphical analysis
The protein synthesis rate estimated from the earlier work involved analysis of blood sampled from the radial artery into free 11C-MET and 11C-MET which was bound to hemoglobin. This information was not available to us in this study. Thus, as an alternative method to determine the rate of incorporation of 11C-MET into bound skeletal muscle protein, we employed Patlak analysis [13], which can be used to determine the fractional extraction rate Ki from plasma into a peripheral compartment (bound protein). The method assumes that the tissue compartment is in rapid equilibrium with the plasma and bound peripheral compartments, and that release from the bound compartment is not observed in the timescale of the study. To derive Ki from the dynamic images, the ratio TAC(t)/Cp(t) is plotted against ∫Cp(t)dt/Cp(t), integrated between t=0 and t. When the system achieves equilibrium between the three compartments the Patlak plot becomes linear and the Patlak slope Ki can be derived from fitting that linear component. The Patlak slope can be written in terms of the rate constants of the three-compartment model:
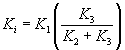
To determine the relationship between Ki and the protein synthesis rate estimated by Fischmann et al. [9], we calculated the Ki based on the individual compartmental rate constants (K1,K2,K3) tabulated for the 6 subjects studied and plotted these values against the tabulated PSR. We observed a strong high linear correlation (r=0.92) between the calculated Patlak slopes and the measured PSR, indicating that Ki could be a useful surrogate measure for PSR.
Demographic and strength data are summarized in Table 1. 11C-MET showed strong uptake in mid thigh skeletal muscle in all of the subjects.
| Sex | Age (years) | Wt (kg) | Ht (m) | Leg Extension Strength(N) | Leg Flexion Strength (N) |
|---|---|---|---|---|---|
| F | 30 | 63.50 | 16.76 | 133.50 | 97.90 |
| F | 31 | 82.55 | 16.26 | 173.55 | 106.80 |
| F | 36 | 52.16 | 16.51 | 120.15 | 89.00 |
| F | 27 | 44.45 | 15.49 | 133.50 | 97.90 |
| F | 27 | 83.91 | 17.53 | 173.55 | 115.70 |
| F | 25 | 113.40 | 18.03 | 146.85 | 80.10 |
| F | 27 | 49.90 | 15.75 | 146.85 | 71.20 |
| F | 75 | 56.70 | 15.75 | 160.20 | 89.00 |
| F | 70 | 53.07 | 15.49 | 102.35 | 71.20 |
| F | 76 | 74.84 | 16.51 | 115.70 | 57.85 |
Table 1: Demographic variables and strength measurements from study subjects.
Exercise effects-qualitative results
The effect of unilateral leg exercise on uptake of 11C-MET in skeletal muscle varied with the time between completion of exercise and injection of 11C-MET. The first five subjects were imaged 2-3 hours after exercise. Despite evidence from the biopsy literature indicating an elevated PSR at least two hours after exercise, we observed no differences between the exercised and control limbs. The second 5 subjects were imaged one hour post-exercise. In contrast, these subjects showed a clear enhancement of 11C-MET uptake in the exercised limb. Figure 5 compares axial mid-thigh PET cross-sections in subjects imaged at three and one-hour post exercise.
Results of Patlak and three compartment analyses
Patlak slopes Ki and individual compartmental rate constants (K1,K2,K3) are tabulated for the exercised and control limbs for the ten subjects in Table 2. To illustrate the variations between the subjects, the Ki are plotted for the two limbs for each subject in Figure 6. In the subjects imaged over 2 hours after exercise, the differences in Ki between the exercise and control legs differences range from -2.4% to 4%. In contrast, subjects imaged 1 hour after exercise have uniformly higher Ki in the exercised limb, ranging from 7% to 60%. The Ki values measured for the exercised limb in the three older subjects were 30%- 50% lower than the values measured for the exercised limb in the two younger subjects imaged one hour after exercise, and these subjects had Ki values comparable to those of the younger subjects imaged 2-3 hours after exercise. Although all of the older subjects showed relatively low values of Ki, one of the three subjects showed a large enhancement of protein synthesis in the exercised limb (60%) and the other two showed small relatively small enhancements (7% and 9%). Finally, inspection of the compartmental rate constants in Table 2 show that vb (fractional blood volume) and K1, (rate constant for extraction of 11C-MET from plasma to tissue) but not K2 and K3 (rate constants for 11C-MET release from tissue to plasma and incorporation from tissue to bound protein), are larger in the exercised than in the control limb.
| cm3/g/min | 1/min | 1/min | cm3/g/min | |||||||
| AGE-HRS | vb-C | vb-EX | K1-C | K1-EX | k2-C | k2-EX | k3-C | k3-EX | Ki-C | Ki-EX |
| 29-2 | 0.0701 | 0.0679 | 0.0281 | 0.0271 | 0.0746 | 0.0738 | 0.0245 | 0.0247 | 0.0062 | 0.0061 |
| 30-2 | 0.0493 | 0.0481 | 0.0874 | 0.0884 | 0.2014 | 0.1900 | 0.0320 | 0.0306 | 0.0146 | 0.0151 |
| 36-2 | 0.1810 | 0.1595 | 0.1157 | 0.1239 | 0.3294 | 0.3113 | 0.0583 | 0.0470 | 0.0152 | 0.0140 |
| 26-2 | 0.0849 | 0.0821 | 0.0196 | 0.0201 | 0.0367 | 0.0384 | 0.0114 | 0.0117 | 0.0056 | 0.0055 |
| 25-2 | 0.0445 | 0.0422 | 0.0890 | 0.0840 | 0.2440 | 0.2505 | 0.0265 | 0.0266 | 0.0125 | 0.0115 |
| 27-1 | 0.0574 | 0.0651 | 0.1533 | 0.1786 | 0.3945 | 0.3720 | 0.0708 | 0.0734 | 0.0305 | 0.0387 |
| 27-1 | 0.0889 | 0.1481 | 0.2955 | 0.4471 | 0.2542 | 0.2697 | 0.0441 | 0.0479 | 0.0523 | 0.0760 |
| 75-1 | 0.0533 | 0.0829 | 0.0798 | 0.1227 | 0.2836 | 0.2917 | 0.0246 | 0.0247 | 0.0084 | 0.0122 |
| 70-1 | 0.0577 | 0.0667 | 0.1124 | 0.1358 | 0.4286 | 0.4314 | 0.0272 | 0.0259 | 0.0078 | 0.0089 |
| 76-1 | 0.0356 | 0.0358 | 0.0845 | 0.0853 | 0.5231 | 0.5051 | 0.0485 | 0.0484 | 0.0076 | 0.0079 |
Table 2: Rate constants calculated from dynamic images and Patlak slopes for subjects. EX and C represent exercise and control limbs respectively.
Consistent with earlier work [9], our results showed clearly delineated uptake of 11C-MET into the muscle of the thigh. The Patlak slope Ki, a quantitative measure of extraction of the substrate from plasma to the bound compartment in muscle, agreed with the Ki calculated from the kinetic parameter estimates from Fischman et al. [9]. The Ki derived from the control legs of our five subjects imaged at 2-3 hours post exercise, which represented the closest equivalent in our data to resting state measures, had a mean value of 0.0108 cm3/ min/g compared to 0.013cm3/min/g for the 6 subjects imaged in the earlier work [9]. Thus, despite differences in image data acquisition and processing, including use of newer generation radiosynthesis and PET imaging technology, as well as derivation of the arterial input function from the dynamic images rather than from an arterial line, our qualitative and quantitative results were in agreement with the initial published study [9].
The goal of our exploratory study was to determine if this imaging and analytic approach could detect the effect of unilateral leg extension and flexion exercise in a group of young and elderly women. We hypothesized that the exercised leg would show increased incorporation of 11C-MET into skeletal muscle tissue. In order to rule out increased blood flow as a factor in the increased uptake of 11CMET, we carried out the imaging after delays of 1-3 hours, based on evidence that blood flow to the lower extremities returns to baseline values minutes after completion of exercise [14]. Because arterial line measurements of Hsu et al. [10] and Fischmann et al. [9] were not available to us, we used as a measure of PSR the Patlak slope Ki, which measures the fractional transport of 11C-MET from the plasma to the bound protein compartment, having established the strong correspondence between Ki and PSR from the data of Fischman et al.
We observed a unilateral exercise response at 1 hour but not 2-3 hours post-exercise, as manifested both visually and by differences in Ki. The timing of the unilateral response of Ki corresponded to those observed for the blood volume fraction vb and the rate constant K1 for extraction from plasma to muscle tissue. The observation of a side to side difference at one but not 2-3 hours may potentially be explained by the fasting state conditions in which our exercise and imaging study was carried out. The combination of exercise and the fruit drink may have resulted in a transient increase in circulating insulin levels, thus increasing capillary recruitment [15] and insulin-dependent transport of methionine [16] across the muscle cell membrane, which would be consistent with the acute side to side differences in blood volume fraction vb and the K1 rate constant. Our observed response seems inconsistent with previous studies of acute response to unilateral resistance exercise carried out using biopsies, which show continued elevation of protein synthesis several hours after exercise [17]. However, it should be noted that these biopsy studies involve infusions of supraphysiologic doses of amino acids that last hours and are even continued after exercise, whereas our experiment introduced the amino acid as a single low dose bolus. Thus it may not be possible to directly compare our results to results obtained with biopsy techniques. Future investigations of our approach should be carefully designed in parallel with biopsy studies to best establish the correspondence between these two methods.
The data obtained at one hour post exercise included images from three elderly subjects, who, similar to the two young subjects, showed side to side differences in 11C-MET uptake. However, these three subjects showed much lower (400-700%) lower values of Ki than the two younger subjects imaged at the same time point. The direction, if not the magnitude, of these differences are consistent with evidence in the skeletal muscle biopsy literature of attenuated response of PSR to chronic [18] and acute exercise [19,20] in older compared to younger subjects. These results appear to depend on whether the exercise was done in the fasting or fed state, with severe attenuation of protein synthesis in the elderly in the fasted state [19], and a delayed but strong response in the fed state [20].
Dynamic PET/CT imaging of 11C-MET uptake can offer distinct and complementary information compared to biopsy assays of uptake of phenylalanine, leucine and other substrates typically used to study amino acid kinetics, which involve systemic or local infusion of an amino acid coupled to a stable isotope tracer, followed by extraction and subsequent assay of the muscle tissue [21-23]. PET/CT imaging can be used to obtain spatial maps of amino acid kinetics within whole muscle beds as opposed to the extremely small tissue sub-volumes assessed by biopsy. In the clinical research setting, the kinetic information can be used to study, under controlled conditions, highly specific physiologic stimuli such as exercise and drug interventions that might differentially affect rate constants that model specific processes, such as cell barrier transport, local blood flow or activation or suppression kinases associated with protein synthesis. When combined with CT, this approach can be used to integrate a measure of tissue metabolism with measures of anatomy and composition that are often assessed in research studies. However, this approach also has limitations. Although it is relatively non-invasive, 11C-MET imaging entails a 4mSv effective dose of radiation (3.6 mSv corresponds to the equivalent of 1 year of background radiation). Further studies will be required to determine how much this radiation dose can be reduced. Additionally, the radiosynthesis of 11C-MET requires an onsite cyclotron equipped with a radiochemistry facility. The approach is thus more suited to the clinical research environment of a university research center, where such resources are available. Finally, this approach cannot replace biopsy measures, which can be used to carry out a wide range of cellular and molecular studies of muscle, such as determining the distribution of the label between different protein compartments or carrying out gene expression studies. However, biopsy measures are highly painful and invasive, and a limited number of samples can be taken from a subject at a given time. Thus, the ability of 11C-MET to characterize amino acid kinetics and protein synthesis rate can allow researchers to focus biopsy measurements on measures that may provide more physiologic insight.
In summary, we have found that 11C-MET imaging to determine the rate of incorporation of labeled methionine into skeletal muscle protein, both qualitatively and quantitatively, is responsive to an exercise stimulus, and appears to decrease with advancing age. The anatomic clarity afforded by a modern volumetric PET/CT imaging system has the potential to open up protein synthesis measurements to whole muscles, including postural muscles and deep muscles currently inaccessible to biopsy studies. Despite limitations described above, this research represents a potentially important methodological advance that could be of great interest to clinical research in the area of agerelated sarcopenia and other muscle wasting conditions that affect our population.
The authors would like to thank Michelle Swenson and Michael Moles for technical assistance with the PET/CT scanning. The study utilized exercise equipment built with funding from the National Space Biomedical Research Institute Grant BL-01301. This work was supported by Merck under the Investigator-Initiated Science Program, Grant IISP-37004.
None