Journal of Thyroid Disorders & Therapy
Open Access
ISSN: 2167-7948
ISSN: 2167-7948
Research Article - (2014) Volume 3, Issue 3
Context: There is little consensus on the most appropriate dose regimen for radioiodine treatment (RIT) of Graves’ hyperthyroidism (GH), and quantitative studies on the role of the efficacy-affecting parameters are lacking.
Objective: This prospective study was designed to evaluate the feasibility of quantitative RIT of GH using a modified formula, which took into account eight parameters associated with the outcome.
Design: A 1-year follow-up study of 205 GH patients was performed.
The administered activity of 131I was established using a formula,
Activity MBq = [(gland weight (g) ×in tended activity) (MBq/g) /Max uptake] (% )× (1 + X/30) in which the X represented the sum of the parameters, and the administered activity increased or decreased by 1/30 when each of the parameters was present or absent. The subjects were randomized into two groups according to the intended activity.
Results: No significant differences at baseline were noted between the two groups. The mean administered activity of 131I in 3.7 MBq/g group was significantly lower than that in 5.55 MBq/g group. One year after therapy, 77.6% patients were treated successfully, with 76.3% in the 3.7 MBq/g group (58.8% achieved euthyroidism, 17.5% became hypothyroid) and 78.7% in the 5.55 MBq/g group (38.9% achieved euthyroidism, 39.8% became hypothyroid). Hypothyroidism occurred earlier in the 5.55 MBq/g group with rates significantly higher than those in the 3.7 MBq/g group.
Conclusions: The modified formula seems feasible in the RIT of GH, which takes into account the quantification of efficacy-affecting parameters. The intended activity of 5.55 MBq/g may result in a higher rate of hypothyroidism and an earlier cure of hyperthyroidism than the activity of 3.7 MBq/g, which appears to be more favorable for maintaining euthyroidism.
Graves’ disease (GD) is the leading cause of hyperthyroidism, affecting approximately 0.5% of the population, and is more common in women aged 20-40 years [1]. Data on the epidemiology of Graves’ hyperthyroidism (GH) in shows that the total morbidity rate is 3% (4.1% for females and 1.6% for males). GH has adverse effects on virtually all organ systems, especially cardiac and skeletal health, and is more common in older patients [2,3]. Severe hyperthyroidism induces a variety of liver function abnormalities, heart failure, respiratory muscle paralysis, thyroid crisis and even death [4,5].
At present, several therapeutic options are available for the treatment of GH, including antithyroid drug (ATD) medication, near-total resection, and Radioiodine Therapy (RIT). Although radioiodine (131I) is becoming more and more popular as first-line therapy for GH in western countries because of its safety, convenience, and effectiveness, there is still no general consensus on the choice of the best therapeutic regimen among empirical, fixed activity, and calculated activity methods [6-8]. The administration of a fixed activity may lead to too much or too little radioactivity being delivered; that is, unnecessary exposure for the patient and for the environment or, conversely, insufficient to achieve a cure. Even when a calculated activity method is used with respect to thyroid weight and 24-h 131I uptake, high incidences of hypothyroidism following RIT have been noted, which severely prohibits the extensive application of RIT in GH patients in China and other countries. Studies on the optimal strategies for RIT which are focused on the intended activity of radioiodine per gram of thyroid weight are ongoing. Currently, intended activities which vary narrowly from 2.59 to 4.44 MBq/g are recommended by the Chinese Association of Nuclear Medicine, and are much lower than those recommended by the ATA and AACE [9]. Therefore, exploring higher intended activities are meaningful for clinicians to expand the knowledge on RIT in Chinese patients with GH.
Moreover, it has been reported that the outcome of RIT is associated with several factors besides thyroid weight and 24-h 131I uptake, such as patient’s age, coexisting thyroid nodules, prior anti-thyroid drugs, duration of hyperthyroidism, severity of hyperthyroidism, titer of thyroid-stimulating hormone receptor antibodies (TRAb), iodine uptake peak, and complications. The optimal approach is now regarded as the administration of a computed therapeutic activity that takes into account such individual parameters [3,10-18]. However, quantitative studies on the role of the above parameters which influence therapeutic effect have not been carried out to date, and subjectivity cannot be avoided in practice.
We, therefore, conducted this study in Chinese patients with GH to compare the outcomes of RIT using different intended activities (3.7 MBq/g or 5.55 MBq/g) and to explore the feasibility of a hypothesized weighting factor of 1/30 for each of the parameters affecting efficacy of RIT.
Patients
From April 2009 to August 2011, patients with GH, who were randomized to undergo RIT, were enrolled and analyzed in the present study. The diagnosis was confirmed by clinical, laboratory, ultrasonographic, and RAI uptake results in all patients. Patients who had a history of RIT were excluded from our study. Prior to the 131I uptake test, all patients were asked to refrain from eating iodinated food or drugs for 1 month, and administration of methimazole or propylthiouracil was discontinued for not less than three days and two weeks, respectively. Approval for the protocol was received from the ethics board before the beginning of the study. All subjects gave written informed consent for participation in the study.
Biochemical evaluation
One week before RIT and at 2, 4, 6, and 12 months after therapy, complete measurements of thyroid-stimulating hormone (TSH), free T3 (FT3), free T4 (FT4), thyroglobulin antibody (TGAb), and thyroid peroxidase antibody (TPOAb) were performed using a chemiluminescent immunoassay system (Immulite, Diagnostic Products Corp, Los Angeles, CA, USA). Reference ranges were 0.27-4.20 mIU/l for TSH, 3.10-6.80 pmol/l for FT3, 12.00-22.00 pmol/l for FT4, 0.00-115.00 KIU/l for TGAb, and 0.00-3.5 KIU/l for TPOAb, respectively. TRAb was measured using ELISA with the reference range of 0-2 U/l. A pregnancy test was obtained and a negative result was verified within 48 hours prior to RIT in female patient with childbearing potential.
Thyroid weight
All patients were examined by the same operator (Dr. Tao Ying) using a HI Vision Preirus scanner (5-13 MHz linear array, HITACHI Medical Corporation, Tokyo, Japan). The information provided by ultrasonography included the three-dimensional sizes of the thyroid, blood flow and nodules. Volume of the thyroid was calculated according to the ellipsoid model [length (cm) × width (cm) × depth (cm) × π/6] [19]. The thyroid gland weight was calculated, assuming 1 ml corresponded to 1 g tissue.
131I test and therapy
The radioiodine uptake test was carried out 3 h and 24 h after oral administration of 0.185 MBq 131I in liquid using a collimated scintillation probe in patients compared to a neck phantom, and corrected for the background radiation. 131I uptake which peaked at 3 h was defined as early iodine uptake. Once 24 h uptake was completed, the therapeutic activity of 131I was calculated and 131I-labled sodium iodide (Na131I) in liquid was immediately administered orally as a single dose.
The administered activity was calculated according to a modified formula:
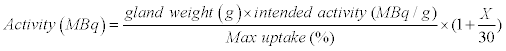 , in which gland weight was estimated by ultrasonography as described above. The X factor represented the sum of the eight parameters associated with the outcome.
, in which gland weight was estimated by ultrasonography as described above. The X factor represented the sum of the eight parameters associated with the outcome.
The administered activity of 131I increased or decreased by 1/30 when each of the eight parameters (patient’s age over 30 years, coexisting thyroid nodules, prior anti-thyroid drugs, duration of hyperthyroidism longer than 1.5 years, level of FT3 or FT4 higher than 3-fold of the upper limit of the normal range, titer of TRAb over 2 U/l, early iodine uptake peak, and any complication) was present or absent.
The patients were randomized into two groups according to the intended activity as either 3.7 MBq/g or 5.55 MBq/g.
Follow-up
The patients were followed up at 2, 4, 6, and 12 months after treatment, using a questionnaire to inquire about possible symptoms of hyperthyroidism or hypothyroidism. Thyroid function tests (FT3, FT4, and TSH) and ultrasonography examinations were required at each visit. Outcomes which included euthyroidism, hypothyroidism, and hyperthyroidism were registered at each follow-up visit for both treatment groups. Thyroid hormone replacement was determined by the results of thyroid function tests, clinical symptoms, and physical examination. After RIT, patients with severe hyperthyroidism were treated with beta-adrenergic blockades, and followed by a second course of RIT one year later if persistent or recurrent hyperthyroidism was verified.
Statistical analyses
Data analyses were carried out using SPSS 13.0. The Mann-Whitney test was used to compare non-normally distributed data, including age, thyroid weight, TSH, FT4, FT3 131I uptake, and administered activity between the two groups of patients. The Student's t-test was used for the comparison of normally distributed data (TRAb). The chi-square test was used to compare qualitative variables, including gender, euthyroidism, hypothyroidism, and hyperthyroidism during the follow-up. Two-sided tests were used and P < 0.05 was considered statistically significant.
Baseline characteristics
Two hundred and five patients completed the last follow-up visit at the end of the study in 2012, in which 61 (29.8%) patients were male and 144 (70.2%) were female. Age ranged from 18 to 69 years with a mean of 39.1 ± 12.6 years. Thyroid weight ranged from 24 to 68 g with a mean of 42 ± 11.4 g. 131I uptakes at 3 h and 24 h ranged from 45% to 86% with a mean of 61.4 ± 9.8% and 53% to 95% with a mean of 69.6 ± 8.9%, respectively.
An early 131I uptake peak was noted in 60 (29.3%) patients with a mean of 73 ± 18.6%. A summary of the baseline characteristics of the patients according to intended activity are shown in Table 1 mainly as the mean ± SD. Except for administered 131I activity, there were no significant differences between the two groups regarding age, sex ratio, thyroid weight, thyroid function, TRAb, and 131I uptake. The mean administered activity of 131I in 3.7 MBq/g group was 140.6 ± 42.2 MBq, which was significantly lower than that in 5.55 MBq/g group with the mean of 244.2 ± 64.8 MBq (Z = 10.257, P < 0.01).
| Characteristic | Intended activity (MBq/g) | P | |
|---|---|---|---|
| 3.7 | 5.55 | ||
| Number of patients | 97 | 108 | - |
| Age (y) | 37.9 ± 12.4 | 40.1 ± 12.6 | 0.301 |
| Gender (female/male) | 67/30 | 77/31 | 0.782 |
| Thyroid weight (g) | 41.3 ±11.3 | 42.9 ±11.5 | 0.303 |
| TSH (mIU/L) | 0.03 ± 0.04 | 0.04 ± 0.04 | 0.247 |
| FT4 (pmol/L) | 51.3 ± 13.0 | 55.3 ± 16.6 | 0.108 |
| FT3 (pmol/L) | 24.1 ± 9.0 | 24.7 ± 9.4 | 0.542 |
| TRAb (U/L) | 20.3 ± 6.0 | 19.8 ± 5.7 | 0.614 |
| 3-h uptake (%) | 60.5 ± 9.4 | 62.4 ± 10.1 | 0.269 |
| 24-huptake (%) | 69.8 ± 8.8 | 69.4 ± 8.9 | 0.665 |
| Administered activity (MBq) | 140.6 ± 42.2 | 244.2 ± 64.8 | 0.000 |
Table 1: Baseline characteristics of patients with Graves’ hyperthyroidism according to intended activity
Outcomes at one year post RIT
Totally, one hundred and fifty-nine (77.6%) of 205 patients were successfully treated according to the final follow-up at one year post RIT, including patients who achieved euthyroidism and hypothyroidism. Success rates were 76.3% (74/97) in the 3.7 MBq/g group and 78.7% (85/108) in the 5.55 MBq/g group, respectively. As is shown in (Figure 1), at one year after receiving the intended activity of 3.7 MBq/g, fifty-seven patients (58.8%) achieved euthyroidism, seventeen patients (17.5%) became hypothyroid, and 23 patients (23.7%) remained hyperthyroid. However, following treatment with the intended activity of 5.55 MBq/g, forty-two patients (38.9%) achieved euthyroidism, forty-three patients (39.8%) became hypothyroidism, and 23 patients (21.3%) remained hyperthyroidism. The rates of euthyroidism and hypothyroidism were statistically different between the two groups (The value of X2 was 8.08 and 12.26, respectively, P < 0.05). Although the rate of hyperthyroidism in the intended activity of 5.55 MBq/g group was lower than that in the 3.7 MBq/g group, this was not statistically different (X2 = 0.17, P > 0.05).
Euthyroidism during the follow-up
As is shown in Figure 2, percentages of patients who achieved euthyroidism at 2, 4, 6, and 12 months after RIT increased in the 3.7 MBq/g group, and were 3% (3/97), 21.6 % (21/97), 44.3 % (43/97), and 58.8 % (57/97), respectively. Whereas, 6.5% (7/108), 35.1% (38/108), 37.0% (40/108), and 38.9% (42/108) patients were found with normal thyroid function in the 5.55 MBq/g group at the same time points, respectively.
Hyperthyroidism during the follow-up
Figure 3 shows decreasing rates of patients with hyperthyroidism noted in both groups during the follow-up. Following treatment with the intended activity of 3.7 MBq/g, the total numbers of patients, who were identified with hyperthyroidism at 2, 4, 6, and 12 months after 131I treatment, were 93 (96%), 72 (74.2%), 40(41.2%), and 23 (23.7%), respectively. Correspondingly, using the intended activity of 5.55 MBq/g, the total numbers of patients were 96 (88.9%), 47 (43.5%), 28 (25.9%), and 23 (21.3%), respectively. During the follow-up, 58.8% (57/97) patients achieved success in sixth month after RIT using the intended activity of 3.7 MBq/g, while 56.5% (61/108) patients achieved success in 4 months using the intended activity of 5.55 MBq/g.
Hypothyroidism during the follow-up
In all, sixty (29.3%) of 205 patients became hypothyroid according to follow-up at one year post RIT. Figure 4 shows the increasing rates of patients with hypothyroidism in both groups during the follow-up. Following treatment with the intended activity of 3.7 MBq/g, the total number of patients, who became hypothyroid at 2 months, 4 months, 6 months and 12 months after RIT, was 1 (1%), 4 (4.1%), 14(14.4%), and 17 (17.5%), respectively. Correspondingly, following treatment with the intended activity of 5.55 MBq/g, the total number of patients who became hypothyroid was 5 (4.6%, X2 = 1.23, P > 0.05), 23 (21.3%, X2 = 13.18, P < 0.05), 40 (37%, X2 = 13.4, P < 0.05), and 43 (39.8%, X2 = 12.2, P < 0.05), respectively. Although the rates of hypothyroidism noted at 2 months post RIT between the two groups were not significantly different, the rates of hypothyroidism observed at 4, 6, and 12 months after RIT using the intended activity of 5.55 MBq/g were significantly higher than those using the intended activity of 3.7 MBq/g. In terms of diagnosis, 29.8% (61/205) patients had both GD and Hashimoto’s thyroiditis (HT) and 70.2% (144/205) patients had GD only. 19.7% (12/61) patients with both GH and HT and 20.8% (30/144) patients with GH only were hypothyroid at one year RIT (X2 = 1.03, P > 0.05).
RIT has been widely used in the treatment of GH as either the first-line or second-line therapeutic strategy for more than seven decades [20,21]. This treatment is quite effective, provided sufficient radiation is deposited in the thyroid, which can be accomplished by sodium iodide symporter-mediated accumulation and TPO-mediated organification of radioiodine. However, the amount of radioiodine activity to administer can differ greatly among institutions and practitioners, depending on the aim of the treatment, the method used, and factors affecting the efficacy of RIT.
Many experts believe that the goal of RIT is to control hyperthyroidism by rendering the patient hypothyroid, and obtaining sustained euthyroidism appears to be a futile objective. However, striving for euthroidism to avoid lifelong levothyroxine substitution is of great value, since the best outcome of RIT for GH patients is to recover normal thyroid function, without relapse or hypothyroidism. Over the last few decades, much attention has been focused on achieving euthyroidism by adjusting the intended activity to identify an optimum dosing strategy [22]. We believe, in line with others, that the optimal approach should be to eradicate hyperthyroidism at the lowest effective radioiodine activity; that is, with an activity calculated by using personalized dosimetry [23,24].
As demonstrated in our study, the protocol we used effectively treated hyperthyroidism and significantly reduced the hypothyroidism. The total cure rate observed at one year post RIT, including euthyroidism and hypothyroidism, was 77.6% in all patients. However, only 29.3% of patients were identified as hypothyroid. The total success rate was a little lower, whereas the rate of hypothyroidism was much lower than those using fixed activities of 370 MBq and 450 MBq, which resulted in hypothyroidism in 69% and 75% patients, respectively [25,26].
Our study also showed that both the administered activity and the rate of euthyroidism and hypothyroidism were statistically different between the 3.7 MBq/g intended activity group and the 5.55 MBq/g intended activity group. A higher incidence of hypothyroidism was noted following treatment with the intended activity of 5.55 MBq/g, whereas a higher rate of euthyroidism was found in the relatively low intended activity group, indicating that the intended activity of 3.7 MBq/g may be more favorable for achieving euthyroidism than the intended activity of 5.55 MBq/g. In addition, no statistical difference in the rates of hyperthyroidism at one year post treatment was found between the two groups, in accordance with the similar cure rates (76.3% vs. 78.7%). Therefore, it may be deduced that both intended activities of 5.55 MBq/g and 3.7 MBq/g produce similar cure rates if follow-up is long enough to be one year. With respect to the increasing rates of hypothyroidism we observed during the one year follow-up, the rates of hypothyroidism observed at 4, 6, and 12 months after RIT with the intended activity of 5.55 MBq/g were significantly higher than those with the intended activity of 3.7 MBq/g. It seems that the intended activity of 5.55 MBq/g may induce a higher rate of hypothyroidism and an earlier cure for hyperthyroidism.
The outcomes were determined according to the patients’ status during the one-year follow-up. Some reports suggested that hypothyroidism may present in 24 to 60% of GH patients after 20-24 years, and is more commonly found in patients who also have HT [27]. Compared with patients with only GH, a higher incidence of hypothyroidism was not found in patients with both GH and HT in our study, which was possibly due to the limited follow-up period of only 12 months. It can be presumed that prolonged follow-up would have resulted in a higher proportion of patients with hypothyroidism, because with continuous autoimmune destruction which occurs with GH, thyroid hormones may slowly decrease to normal or even lower concentrations.
Compared with the incidence of hypothyroidism at six months post treatment of 14.4% in patients administered the intended activity of 3.7 MBq/g, the symptoms of hyperthyroidism were rectified within a shorter time in the 5.55 MBq/g group. In the 5.5 MBq/g group, about half of the patients who needed thyroid hormone replacement at the end of follow-up were hypothyroid in 4 months, when 21.3% patients were identified with hypothyroidism by biochemical evaluations. This may be explained by the fact that although the full effect of the RAI might take at least 6 months, successful therapy is generally observed by a significant decline in free T4 and T3 with a decrease in goiter size within 3 months after RIT [28]. Thus, we recommend that thyroid function tests should be carried out every 2 months or more frequently for at least 6 months, and thyroid hormone replacement should be started as soon as the peripheral thyroid hormone levels decline below the normal range, even if the TSH is still suppressed.
Although it was not the aim of this study to decide on the goal of 131I treatment, it is important to consider whether the main goal of 131I therapy should be long-term euthyroidism or a cure for hyperthyroidism. If the main goal is a cure for hyperthyroidism, higher intended activities may be used in order to prevent a prolonged hyperthyroid state. In contrast, if the goal is the adequate treatment of hyperthyroidism, lower intended activities should be used aiming to prevent of hypothyroidism in a large proportion of patients. Therefore, our findings are useful for selecting a high intended activity such as 5.5 MBq/g or more for treating GH patients with cardiovascular problems or other comorbidities, in which an early cure for hyperthyroidism is needed. Otherwise, using a lower intended activity such as 3.7 MBq/g might be more favorable for maintaining euthyroidism for a long period.
It has been reported that several factors may contribute to the treatment outcome of GH besides thyroid weight and 24-h 131I uptake, such as patient’s age, coexisting thyroid nodules, prior anti-thyroid drugs, duration and severity of hyperthyroidism, titer of TRAb, iodine uptake peak, and complications [3,10-18]. However, the role of such parameters has not been quantitatively investigated. In our study, we initially used the modified calculated activity formula, taking into account the quantification of eight efficacy-affecting parameters of RIT for GH, to determine the final administered activity of 131I. According to the therapeutic efficacy we observed, the hypothesized weighting factor of 1/30 for each of the above parameters is practical and feasible. The modified formula we proposed quantified each of the efficacy-influencing parameters as 1/30, which effectively avoided subjectivity. This modification may also be useful for unifying the activity standard during the course of RIT for GH.
Since the extent of the impact of the parameters on the efficacy of RIT may be different, our study using the same hypothesized weighting factor of 1/30 for each parameter is still preliminary. According to the outcomes at one year post RIT, forty-six (22.4%) of 205 patients remained hyperthyroid and more courses of RIT were needed, indicating that even lower failure rates should be pursued. This may be due to the negligence of effective half-life of the radioiodine and the thyroid absorbed dose value using MIRD algorithm. We admit that if the parameters mentioned in our study are fully considered, there is a small overlap between the upper range of the 3.7 MBq/g and the lower range of 5.55 MBq/g. Well-designed studies with a larger sample size and longer follow-up are still needed.
The modified formula,
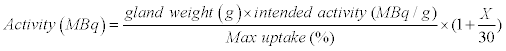 , is feasible in the RIT for GH with the advantage of the quantification of efficacy-affecting factors. Although the difference in treatment success rates at one year post treatment was not significant between the intended activities, the intended activity of 5.55 MBq/g may induce a higher incidence of hypothyroidism and an earlier cure for hyperthyroidism, while the intended activity of 3.7 MBq/g appears to be more favorable for achieving euthyroidism. Acknowledgement This study was sponsored by the National Natural Science Foundation of China (81271609) and Shanghai Rising-Star Program (12QH1401600).
, is feasible in the RIT for GH with the advantage of the quantification of efficacy-affecting factors. Although the difference in treatment success rates at one year post treatment was not significant between the intended activities, the intended activity of 5.55 MBq/g may induce a higher incidence of hypothyroidism and an earlier cure for hyperthyroidism, while the intended activity of 3.7 MBq/g appears to be more favorable for achieving euthyroidism. Acknowledgement This study was sponsored by the National Natural Science Foundation of China (81271609) and Shanghai Rising-Star Program (12QH1401600).