
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2022)Volume 12, Issue 3
Liver injury is a complex heterogeneous metabolic disorder characterized by chronic hyperglycemia, diabetes, abnormalities in lipid and carbohydrate metabolism. Alloxan an oxidation product of uric acid, 2, 4, 5, 6 (1H, 3H)-pyrimidinetetrone, capable of inducing diabetes by destroying pancreatic tissue and has been widely used for induction of experimental diabetes. Quercetin are flavonoids and has been proved to be effective against pancreatic islets in diabetogenic rats modulating glucose homeostasis, insulin-resistance, oxidative injury and cell death. The present study demonstrated the alloxan induced liver injury and and its protection by quercetin administration in adult male rats. Administration of 130 mg/kg alloxan for 28 days induce diabetes associated liver injury as histomorphological alteration includes vacuolar degeneration of hepatocytes, dilated sinusoids and central vein, and focal hepatocellular necrosis and increased level of liver enzymes serum Alanine Aminotransaminase (ALT), Aspartate Aminotransaminase (AST) and Alkaline Phosphatase (ALP) level of antioxidant enzymes such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GSHPx) were decreased. Triglycerides, HDL-c, hepatic glycogen, total protein, and body weight were significantly decreased in alloxan induced diabetic rats. However, increased total bilirubin LDL-c, hyperglycemia and hypercholesterolemia were observed. Administration of 150 mg/kg quercetin in alloxan treated rats significantly ameliorated these effects and potentially reversed the liver injury. Hepato-protective mechanism of quercetin was not yet completely understood.
Hyperglycemia; Quercetin; Alloxan; Hypercholesterolemia; SOD; CAT; GSH-Px; HDL-c
Liver being the largest organ of the body is involved in the production of a variety of plasma proteins required for a number of vital body functions. Liver is a major metabolic house where large numbers of substances are catalyzed and several required molecules are synthesized. It is also an accessary gland of digestive system involved in the production of bile that assists in lipolysis in duodenum [1]. Now a day’s liver cirrhosis all over the world seems to be serious public health problem. The essential and main aim of hepatologists is to prevent people from liver cirrhosis and liver fibrosis [2].
There are many life-threatening pathways have been known that cause liver injury in diabetic patients. Insulin resistance the predominant causative factor that cause hyperglycaemia and compensatory hyperinsulinaemia. As an accumulation of insulinsensitive tissues, the liver is among the essential organs defenseless with the impacts of hyperglycaemia-induce oxidative stress, which may prompt liver tissue damage. This was followed by derangement of protein, carbohydrate and lipid metabolism, thereby leading to increased oxidative stress and further triggering the inflammatory cascade. Both oxidative stress and inflammatory responses act as damaging agents in aggravating the pathological condition of DM [3].
Diabetes Mellitus is a heterogeneous, complex metabolic disorder characterized by hyperglycemia, which is caused by a decrease in insulin production by the pancreas or inability of body for using insulin or both. A hormone that regulates the body glucose level is called insulin. The elevated blood glucose level produces long term harmful effects on different organs, including failure and dysfunction of heart, liver, kidney nerves, eyes and blood vessels [4]. Diabetes causes a various type of complications, including hyperglycemia, obesity dyslipidemia, and hypertension [5]. One of the primitive diseases recognized by humans is Diabetes Mellitus. It was described in Egyptian literature approximately 3000 years ago [6].
Diabetes is associated with long term complications effects on liver, kidneys, eyes and nervous system (Hormones) [7]. There are two types of diabetes complications are macro vascular complications and micro vascular complications. Macro vascular complication includes peripheral artery disease, stroke and coronary artery disease while micro vascular complications include hepapathy, neuropathy, retinopathy and nephropathy [8].
In some cases, DM (Diabetes Mellitus) causes excessive accumulation of fat cells in the liver resulting in a fatty liver and, consequently, NAFLD. Subsequently, 2%–3% of NAFLD patients experience hepatic inflammation, necrosis and fibrosis, which are symptoms of a condition known as Non-Alcoholic Steatohepatitis (NASH). Hepatocellular Carcinoma (HCC) leads to fibrotic liver that result in liver injury [9]. Hepatocellular Carcinoma (HCC) is most commonly diagnosed cancer and cause of death due to cancer worldwide. The growth of HCC is associated with chronic liver inflammation, fibrosis, and its subsequent irreversible cirrhosis [10-12]. Nonalcoholic Fatty Liver Disease (NAFLD) is currently the most common abnormality that is closely linked to obesity, dyslipidemia, insulin resistance, and type 2 diabetes [13].
Administration of various compound and plant extract have shown to protect liver injury in alloxon- induced diabetic rats as Fipronil (FPN) triggered oxidative damage demonstrated by elevated formation of malondialdehyde and nitric oxide and decreased concentration of glutathione and activities of enzymatic antioxidants (Superoxide Dismutase, Glutathione Peroxidase and Catalase) in the hepatic and renal tissues however oral administration of TAU (Taurine) and NAC (N-Acetyl Cysteine), alone or in combination, sufficiently ameliorated and normalized the harmful effects of FPN on serum biomarkers of hepatorenal injury, lipid peroxidation and tissue antioxidants [14]. The extract of (Pueraria tuberosa) significantly decreased blood glucose level, SGOT, SGPT and alkaline phosphates after 14 days of treatment in alloxan induced diabetic rats. It was reported that administration of 1α, 25(OH)2VD3 in alloxan induced diabetic rats increased serum insulin level, normalized the elevated blood glucose level, decreased hepatic glycogen and increased bilirubin in rats [15].
Quercetins are subgroups of flavonoid found in different kinds of propolis. Quercetin glycosides abundantly found in propolis and several foods like fruits and vegetables, mainly in onions, broccoli, apples, tea and red wine [16]. Flavonoids are phenolic phytochemicals; they are important constituents of the nonenergetic part of the human diet and are thought to promote optimal health, partly via their antioxidant effects in protecting cellular components against ROS [17].
Previous literature has shown many quercetins exert effects on glycogen catabolism. It increases glycogenolysis and consequently glucose release, it reduces lactate production and increase oxygen uptake. Quercetin also increases the cytosolic NADP/NADH ratio, as indicated by the reduced lactate/pyruvate ratio [18].
Quercetin has been proved to be effective against pancreatic islets in diabetogenic rats modulating glucose homeostasis, insulin- resistance, oxidative injury and cell death [19]. Quercetin protects organisms against several types of liver diseases and elicits beneficial effects on glucose and lipid metabolism hence, quercetin shows potential for the treatment of glucose and lipid metabolism disorder in Diabetes Mellitus. This flavonoid can effectively attenuate hyperglycemia in alloxan-induced diabetic rats. Considering that glucose and lipid metabolism disorder was the leading cause of diabetes-related complications, its aimed to explore the protective effect of quercetin against glucose metabolism in diabetes [20]. None of previous research reported the hepato protective effect of quercetin in diabetes. The present study was carried out to investigate the hepatoprotective potential of quercetin in alloxan induced diabetic rats.
Experimental animals
20 albino rats (8-10 months old) weighing about 200 ± 20 g were used in this experiment research study. Rats were obtained from KOC University Animal Research Facilities. Rats were maintained at 25°C ± 2°C, surrounding humidity (40%-60%) with 12 hours’ light dark cycles. Animals will be kept in separate cages. Standard pellet diet and fresh drinking water was supplied. Upon completion of dosages rats were slaughtered for histological and serum analysis.
Chemicals and drugs
Different types of chemicals and drugs were used during this experimental study including normal saline, alloxan monohydrate (molecular formula; C4H2N2O, molar mass, 142.07 g/mol, solubility in water 0.29 g/100 ml). Formalin and quercetin purchased from Sigma-Aldrich.
Induction of experimental diabetes
A single intra peritoneal dose of alloxan monohydrate by injection was given to animals (Sigma Chemical Co., Dorst (UK), poole equals 130 mg/kg. Alloxan is a glucose analogue having toxic effect on pancreatic β-cells and selectively destroyed them when administered to rodents. It produced insulin dependent Diabetes Mellitus in rodents [21]. Before induction rats were kept 12 hours in fasting condition. A total of 10 rats were introduced with freshly prepared alloxan monohydrate.
Determination of body weight
The body weight of mice was measured first day and at end of trial using digital weighing balance. Before the determination of weight of animal, a plastic beaker was weighed and reading of balance was set to zero. Then one by one each rat was put in that beaker and weight was checked and noted. Variation in body weight between control group and treated groups was estimated from the obtained data.
Determination of blood glucose
In this experimental works Blood Glucose Monitoring (On Call EZ 11) and Blood Glucose Test Strips (On Call Plus ACON Laboratories, Inc. USA) were used to estimate the blood glucose level.
Liver histology analysis
Some liver sections, intended for histological examination by light microscopy, were immediately fixed in 10% of formalin and processed in a series of graded ethanol solutions. They were then embedded in paraffin, serially sectioned at 5 μm and stained with hematoxylin‐eosin. Four slides were prepared section. All sections were evaluated for the degree of liver injury.
Biochemical analysis
The clear serum obtained after centrifugation was used for the estimation of ALT (U/L), AST (U/L) total serum protein (g/ dL) and total bilirubin [22]. All parameters were measured by automated Bio-lab 310 serum analyzer using biochemical kits.
Determination of lipid profile parameters analysis
Lipid profile parameters includes, triglycerides, total cholesterol, high density lipoprotein cholesterol (HDL-cholesterol) and low density lipoprotein cholesterol (LDL-cholesterol) and liver glycogen were determined in the serum of male albino rats with help of reagent kit (Randox, Laboratories Ltd, UK).
Statistical analysis
The results were expressed as mean ± SE. Statistical analysis was conducted by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test and level of significance was (P< 0.05).
Effect of quercetin on diabetes induced histopathological alterations
Tissue sample from the liver was kept in 10% neutral buffered formalin, dehydrated gradually using ethanol, cleared in xylene, and then embedded in paraffin wax. Different tissue sections (5 μm thickness) were obtained for routine histopathological examination (stained with H&E). Control group showed fairly normal hepatocytes with normal arrangement of hepatic cords. Alloxan treated group showed vacuolar degeneration of hepatocytes, dilated sinusoids and central vein, and focal hepatocellular necrosis. However mild vacuolar degeneration with dilated sinusoids, central vein was observed similar to control in alloxan plus quercetin treated group indicated the protective role of quercetin against alloxan induced hepatotoxicity. The assessment of hepatic lesions was based on the presence of hepatic degeneration and focal necrosis with leukocytic infiltration (Figure 1).
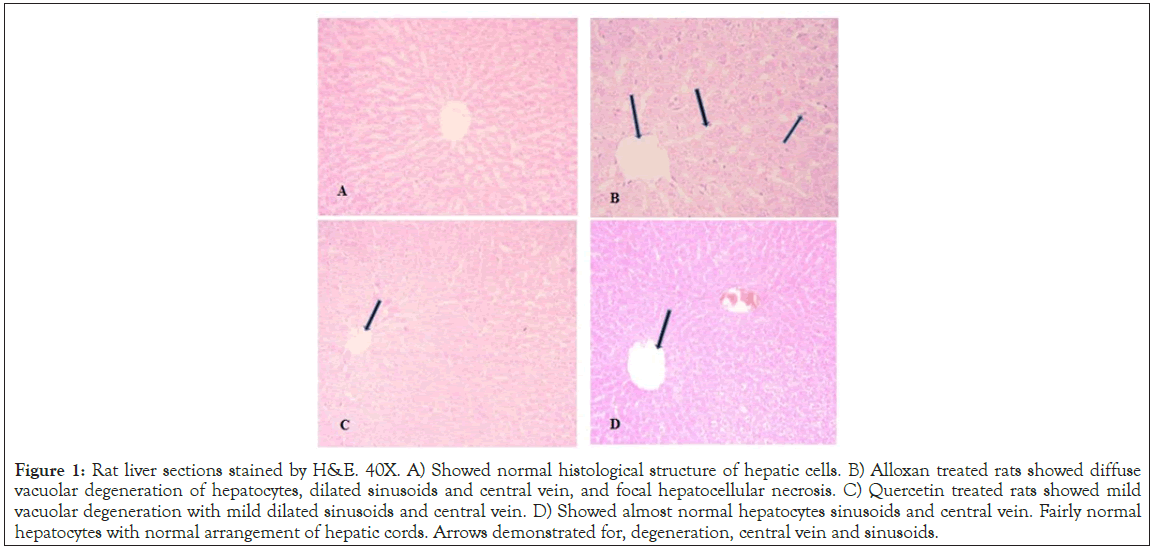
Figure 1: Rat liver sections stained by H&E. 40X. A) Showed normal histological structure of hepatic cells. B) Alloxan treated rats showed diffuse vacuolar degeneration of hepatocytes, dilated sinusoids and central vein, and focal hepatocellular necrosis. C) Quercetin treated rats showed mild vacuolar degeneration with mild dilated sinusoids and central vein. D) Showed almost normal hepatocytes sinusoids and central vein. Fairly normal hepatocytes with normal arrangement of hepatic cords. Arrows demonstrated for, degeneration, central vein and sinusoids.
Effect of quercetin on liver biochemical parameters AST, ALT, ALP and total protein in diabetic rats
Serum AST levels were found significantly (p<0.05) increased in alloxan treated rats compared to control. However, quercetin administration ameliorated the effects. Rats administered with quercetin alone have AST level similar to control (Figure 2). Statistically significant difference was observed in serum ALT levels. However, quercetin administration reversed the hepatotoxic effects of quercetin. Rats administered with quercetin alone has ALT level similar to control. Serum ALP levels were found significantly increased in alloxan treated rats compared to control. However, administration of quercetin counteracts the effects. Rats administered with quercetin alone has ALP level similar to control. The total serum protein content was significantly decreased in alloxan treated rats compared to control. Quercetin administration substantially increased the protein level.
Figure 2: A) Graph showed the ameliorative effect of quercetin against alloxan induced increased AST level. ***P<0.001. B) Graph showed the protective effect of quercetin against alloxan induced increased ALT level in diabetic rats. *P<0.05, **P<0.01 C) Showed the ameliorative effect of quercetin against alloxan induced increased ALP level. ***P<0.001. D) Presented the ameliorative effect of quercetin against alloxan induced decreased protein level. *P<0.05, **P<0.01, ***P<0. 001. Values are represented as mean ± SEM. “a” significant different from control. “b” significant different from alloxan.“c” significant different from alloxan+quercitin.
The decreased antioxidant enzymes level was revered by quercetin in alloxan induced diabetic rats
In the liver homogenates of alloxan‐induced diabetic rats, Catalase (CAT), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) activities were significantly decreased when compared to the controls. The decreased of SOD, CAT and GPx induced by alloxan treatment, was restored to the normal value by quercetin. Supplementation of quercetin in the diet of the (alloxan plus quercitin)‐group enhanced the decreased level of antioxidant enzyme activity which was similar to that of controls as shown in Figure 3.
Figure 3: A) Graph presented the protective effect of quercetin against alloxan induced decreased SOD activity. Significant decreased SOD level shown in alloxan treated group was counteracted by quercitin. **P<0.01, ***P<0.001. B) The protective effect of quercetin against alloxan induced decreased CAT activity. *P<0.05. C) The figure showed the protective effect of quercetin against alloxan induced decreased GPx activity. *P<0.05, ***P<0.001.
Effect of quercetin on cholesterol, triglycerides, HDL-c, LDL-c level and total bilirubin in diabetic rats
The significant (p<0.05) increased serum cholesterol, HDL-c and total bilirubin was shown in alloxan treated rats compared to control. However, the triglycerides and LDL-c level were significantly decreased in alloxan treated rats compared to control. These effects were potentially reversed by quercetin administration along with alloxan as depicted in Figure 4.
Figure 4: A) Graph presented the ameliorative effect of quercetin against alloxan induced decreased triglycerides level, *P<0.05, **P<0.01, ***P<0.001. B) Presented the protective effect of quercetin against alloxan induced increased cholesterol level. ***P<0.001. C) Showed the increased total bilirubin in alloxan induced diabetic rats and its reversal by quercetin. **P<0.01. D) Showed the significant decreased in HDL-c in alloxan induced diabetic rats. Quercetin have showed significantly increased the HDL-c indicated a protective role of quercetin in diabetes. *P<0.05. E) Quercetin have showed significantly decreased LDL-c indicated a protective role of quercetin in diabetes. *P<0.05, **P<0.01.
Hepatic glycogen, blood glucose and body weight evaluations
Hepatic glycogen was significantly decreased in alloxan induced diabetic rats compared to control. Blood glucose level was increased in treated rats and these effects were substantially ameliorated by quercetin. The body weight of alloxon induced diabetic rats was significantly reduced (p<0.05) and was significantly reversed by quercetin treatment as no difference was observed between control and quercetin treated group as depicted in Figure 5.
Figure 5: A) Showed the significant decreased liver glycogen in alloxan induced diabetic rats compared to control and its amelioration by quercetin. *P<0.05. B) Showed the protective effect of quercetin against alloxan induced increased in glucose in diabetic rats. ***P<0.001. C) The protective effect of quercetin against alloxan induced decreased in body weight. Significant decreased in body weight of diabetic group was counteracted by quercetin.
Current study was designed to explore the hepatic injury mediated by diabetes induced by drug alloxan and ameliorative effects of quercetin in alloxan induced liver injury in rats. It has been previously well established that Alloxan caused rats’ β-pancreatic cells destruction in dosage between 150 mg/kg and 200 mg/kg, with the intraperitoneal administration as safety to avoid overall mortality due to its toxicity. Alloxan which was chemically known as an oxidation product of uric acid, 2,4,5,6(1H,3H)-pyrimidinetetrone, potentially damaging pancreatic β cells and has been broadly used for induction of diabetes in experimental rats [23,24]. Being weak acid alloxan was derivative (5-ketobarbituric acid) of barbituric acid therefore rapidly attacks thiol reagents or the sulfhydryl group (-SH) of proteins. The major pathological consequence of alloxan was selective inhibition of glucose-stimulated insulin secretion and was directly linked with the ability of alloxan to oxidized or attack the thiol group present in glucokinase, a glucose phosphorylating enzyme which played a vital role as glucose sensor in the liver and pancreas [21,25].
Quercetin may show medicinal effects due to the presence of phenolic compounds like glycoside, chlorogenic acid, antioxidant and moringinine. Quercetin represented antioxidant and antidiabetic effects. Phytochemical analyses have demonstrated that its leaves are principally contained potassium, calcium, phosphorous, iron, vitamins A and D, essential amino acids, as well as such known antio β xidants such as -carotene, vitamin C, and flavonoids. Glucose 6-phosphate translocase in rat liver was inhibited by chlorogenic acid which may reduce hepatic gluconeogenesis and glycogenolysis. During OGTT chlorogenic acid exhibited a decreased in glycemic response in human [26].
Quercetin may show medicinal effects due to the presence of phenolic compounds like glycoside, chlorogenic acid, antioxidant and moringinine. Quercetin represented antioxidant and antidiabetic effects. Phytochemical analyses have demonstrated that its leaves are principally contained potassium, calcium, phosphorous, iron, vitamins A and D, essential amino acids, as well as such known antioxidants such as β-carotene, vitamin C, and flavonoids. Glucose- 6-phosphate translocase in rat liver was inhibited by Chlorogenic acid which may reduce hepatic gluconeogenesis and glycogenolysis. During OGTT chlorogenic acid exhibited a decreased in glycemic response in human [27].
The results of present study revealed that intragastrically administered of alloxan administration 130 mg/kg alloxan for 28 days induce liver injury as histomorphological alteration includes vacuolar degeneration of hepatocytes, dilated sinusoids and central vein, and focal hepatocellular necrosis also increased level of liver enzymes serum Alanine Aminotransaminase (ALT), Aspartate Aminotransaminase (AST) and Alkaline Phosphatase (ALP) level of antioxidant enzymes such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GSH-Px) were increased also triglycerides and total protein was significantly decreased compared to control whereas cholesterol and serum glucose were significantly increased. There was significant decreased in body weight of treated rats. Variation in hematological parameters as increased in White Blood Cells (WBCs), decreased red blood cells, hemoglobin, and platelets were observed. The results of our study line with the previous study as treated diabetic rats with high dose of quercetin recorded a significant decrease in blood glucose level and a significant increase in insulin [28]. It is due to oxygen radical absorbance capacity, which is probably due to the presence of flavonoids, generally present in large amount in leaves and flowers [29].
The assessment of biochemical markers reveals that quercetin have antidiabetic properties. This may be due to the presence of antidiabetic constituents (glycoside, chlorogenic acid, antioxidant and moringinine). Flavonoids may exert beneficial effects in diabetes by enhancing insulin secretion and reducing apoptosis and promoting proliferation of pancreatic β-cells, improving hyperglycemia through regulation of glucose metabolism in hepatocytes, reducing insulin resistance, inflammation and oxidative stress in muscle and fat and, increasing glucose uptake in skeletal muscle and white adipose tissue [30].
Fipronil (FPN) triggered oxidative damage demonstrated by elevated formation of malondialdehyde and nitric oxide and decreased concentration of glutathione and activities of enzymatic antioxidants (Superoxide Dismutase, Glutathione Peroxidase and Catalase) in the hepatic and renal tissues. Moreover, administration of FPN induced overexpression of the proapoptotic (Bax) and downregulated the expression of the antiapoptotic (Bcl-2) protein. Remarkably, five days prior to and five days along with FPN oral administration of TAU (Taurine) (50 mg Kg-1 BW) and NAC (N-Acetyl Cysteine) (50 mg Kg-1 BW), alone or in combination, sufficiently ameliorated and normalized the harmful effects of FPN on serum biomarkers of hepatorenal injury, lipid peroxidation and tissue antioxidants, our study also agreed with these findings as liver antioxidant enzymes such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GSH-Px) also triglycerides and total protein was significantly decreased in alloxan treated rats compared to control. However, quercetin ameliorated the harmful effects of alloxan [31].
Another study revealed that PTAB (Pueraria tuberosa) significantly decreases blood glucose level in a time dependent manner up to 14 days. As compared to diabetic control, PTAB decreases SGOT, SGPT and alkaline phosphates after 14 days of treatment. In diabetic control, the morphology of liver tissues was found damaged due to deformed hepatocytes and dilated lobules. Most of the hepatocytes after PTAB treatment were comparatively found similar to normal rat tissues, along with dilated blood vessels and normalized liver lobules similar to our findings [32].
Another study has revealed that administration of 1α, 25(OH)2VD3 in alloxan induced diabetic rats increased serum insulin level, normalized the elevated blood glucose level, decreased hepatic glycogen and increased bilirubin in rats [15]. These findings agreed with our study in which quercetin normalized the hyperglycemia, decreased glycogen and increased bilirubin.
The level of total cholesterol, triglyceride, LDL-c and HDL-c in serum after the 14-day sub-chronic study was evaluated in diabetes caused a rise in total cholesterol and triglyceride levels that were reduced by PG (Psidium guajava) administration. Also showed that serum LDL-c levels were significantly increased in diabetic control rats. Treatment of diabetic rats with PG reduced these elevated LDL-c levels to values nearly of normal control. On the other hand, the treatment of diabetic animals with PG caused a significant elevation of HDL-c. Similar findings are revealed in our experiment [33].
The study on alloxan induced diabetic rats revealed that the effect of the ethanolic roots extract of AC (Ageratum Conyzoides) on blood glucose, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT) and hepatic oxidative stress markers ware evaluated in the diabetic rats. The ethanolic roots extract of AC revealed sufficient reduction of blood glucose at the dose of 500 mg/kg when compared with the glibenclamide (600 μg/kg) standard drug. The significant increased level of Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) in the diabetic rats without dose as compared to control group. Furthermore, the levels of oxidative stress markers such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPx), and glutathione (GSH), were decreased significantly in alloxan induced diabetic rats compared to normal rats, while the lipid peroxidation increased in the diabetic group without dose compared to control rats. These finding demonstrated that the morphological, functional and oxidative stress variation in the liver induced by the administration of alloxan in diabetic rats were lesson due to treated ethanolic roots extract of AC similar to quercetin in our experiment [34]. The results compared favorably with that of glibenclamide, a standard drug however, studies are required on a large scale to validate anti diabetic efficacy quercetin and their combination on liver and pancreatic hormone level in another animal model for more significant results.
Conclusively our finding documented that administration of 150 mg/kg quercetin proved a potent therapeutic agent against alloxan induced hepatotoxic effects and significantly reversed the liver injury. The study provided the evident ameliorative effect of quercetin on histology, biochemical and physical aspects of alloxan induced diabetic hepatic injured rats however molecular studies are required to investigate the hepato-protective mechanism of quercetin. Further studies are needed to evaluate potential application and precise mechanism of action for this beneficial Quercetin on amelioration of alloxan induced liver damage in diabetic individuals.
I affirm that the research work is outcome of my own effort, and that I composed article myself. No part of this research has been published in any journal.
I wish to express my sincere gratitude to KOC University Animal Research Facilities, Istanbul Turkey for providing rats for experimental purpose.
Ethical approval for the study was obtained from the Research Ethics Committee of KOC University with numbered 56034207- 514-10.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Aslam M (2022) Quercetin Ameliorated the Alloxan Mediated Hepatic Injury in Diabetic Wistar Rats. J Clin Toxicol. 12:514.
Received: 14-Jul-2022, Manuscript No. JCT-22-18278; Editor assigned: 18-Jul-2022, Pre QC No. JCT-22-18278 (PQ); Reviewed: 01-Aug-2022, QC No. JCT-22-18278; Revised: 08-Aug-2022, Manuscript No. JCT-22-18278 (R); Published: 15-Aug-2022 , DOI: 10.35248/2161-0495-22.12.514
Copyright: © 2022 Aslam M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.