Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2019)Volume 9, Issue 3
Male C57BL/6J (B6) mice aged 24 weeks were provided two concentrations (1.5 or 3.0 g/L) of quercetin glycosides (QG) in drinking water over the subsequent 24 weeks. The objective was to determine if QG could improve motor function during early stages of aging. No sustained significant differences in body composition or food intake were noted to confirm the safety of QG treatment. Treated mice had significantly improved performance in grip strength and rotarod tests but not in treadmill performance. The functional benefits were reflected in muscle morphology with increased wet weights of a selected group of muscles (quadratus femoris, gastrocnemius, tibialis anterior, and soleus) in treated mice. Thus, it would appear that long-term oral treatment with QG can safely and effectively improve selected elements of motor performance and increase muscle mass during the early stages of aging.
Flavonoid; Antioxidant; Oxidative stress; Inflammation; Motor function; Aging
Quercetin, one of the most widely distributed flavonoids, is found in a great variety of foods and is marketed as a nutritional ingredient. The beneficial effects of quercetin have been reported for antioxidant and anti-inflammatory properties. For instance, quercetin treatment can protect neuronal P19 cells from oxidative damage induced by hydrogen peroxide [1]. Using various inducers and markers of oxidative stress, investigators have demonstrated protective effects of quercetin in animals exposed to whole body γ-radiation [2]; chronic stress [3]; and high-fat diets (HFDs) [4]. According to recent reviews, quercetin was reported to exhibit protection against various inflammatory insults in vivo and in vitro [5,6]. The beneficial effects of quercetin include inhibition of various models of inflammation, such as experimental allergic encephalomyelitis [7] and experimental autoimmune myocarditis [8].
Related to possible anti-aging effects, effects of quercetin have been examined on muscle function and atrophy in various animal models. For example, Mukai et al. [9] used a tail suspension model to induce muscle atrophy in mice and reported that quercetin treatment suppressed markers of oxidative stress and attenuated muscle weight loss. In a follow-up study, Mukai et al. [10] evaluated the effects of quercetin in a mouse model of disuse muscle atrophy involving denervation of the sciatic nerve. With 14 days of quercetin supplementation prior to denervation surgery, the level of hydrogen peroxide being produced in the mitochondria from the gastrocnemius muscle was reduced, and AKT phosphorylation was increased, which are important factors in suppressing protein degradation through peroxisome proliferator-activated receptor (PPAR) and nicotinamide adenine dinucleotide (NADH) dehydrogenase 4 expression. Le et al. [11] reported that 9 weeks of quercetin treatment in mice fed HFD lowered levels of inflammatory cytokines and macrophage accumulation in skeletal muscle and attenuated muscle loss and muscle fiber size reduction. Chen et al. [12] used a nerve-crush injury model. Mice treated with quercetin for 35 days exhibited greater sensorimotor recovery from the injury and muscle gene expression regulating the expression of genes involved in regeneration and tropic support were enhanced. Additionally, quercetin increased axon remyelination, motor nerve conduction velocity, and plantar muscle function; all these results suggested a reduction in the degree of distal portion hypotrophy during the peripheral axon regeneration process.
These preclinical findings indicating beneficial health effects of quercetin supplementation have generated clinical trials aimed at different therapeutic targets. The mixed results of these trials have emerged which complicate reaching cogent conclusions. Fortunately, a number of recent meta-analyses have clarified some issues. In their analysis focused on inflammation, Mohammadi- Sartang et al. [13] concluded that quercetin supplementation could reduce serum levels of C-reactive protein, a biomarker of inflammation. Based on their review, Serban et al. [14] concluded that quercetin supplementation could reduce blood pressure; however, their analysis found no significant effects of quercetin on blood lipid levels except for reduced levels of triglycerides at doses above 50 mg/day. In their review, Somerville et al. [15] concluded that quercetin supplementation could improve athletic performance. Thus, the evidence of several meta-analyses involving dozens of controlled human studies suggests the possibility of beneficial effects of quercetin supplementation for a number of health outcomes, including inflammation, cardiovascular function, and athletic performance.
In our current preclinical study, we focused on the effects of quercetin supplementation on motor function and muscle morphology. Departing from the design of many previous studies using young mice (aged <4 months), we chose to initiate quercetin supplementation in fully mature mice (aged 6 months) and evaluate the effects of aging over a period of 6 months and euthanize at 12 months of age for collection of tissues. Shoji et al. [16] reported a major decline in a battery of behavioral tests in C57BL/6J mice between the ages of 2 and 12 months.
Also in contrast to many previous studies, quercetin glycosides (QG) were employed as the treatment, because quercetin itself has a low level of bioavailability and is generally cleared within a few hours [17,18]. QG are alpha-glucosylated derivatives of quercetin with 1-7 glucose moieties on 3-hydroxyl group of quercetin. QG can be efficiently hydrolyzed in the small intestine by β-glucosidases to the aglycone form—and thus can be more readily absorbed into the blood [17,18]. In a recent study employing young mice, Mbikay et al. [19] provided quercetin-3-glucoside in a cholesterol-supplemented diet for 12 weeks and reported reduced plasma cholesterol and insulin in the treated group. In a series of clinical studies, Kanzaki et al. [20-22], investigated the effects of QG in a mixture containing glucosamine and chondroitin sulfate for treatment of knee pain related to osteoarthritis. They found significant improvements compared to placebo following treatment for a number of parameters, including pain and motor function.
In summary, findings from preclinical studies support the therapeutic potential of QG supplementation for the treatment of a variety of conditions related to oxidative stress and inflammation; these studies, however, have been conducted in animal models of these conditions. The current study was conducted to examine effects of QG on aging in motor function and muscle morphology in normal adult mice from a well characterized strain (C57BL/6J).
Animals
Male C57BL/6J (B6) mice were purchased from the Jackson Laboratory, Bar Harbor, ME (Stock Number 000664), aged 4–6 weeks and reared until they reached 24 weeks. The study was conducted within the animal facility of the Pennington Biomedical Research Center (Baton Rouge, LA, USA), which maintains SPF status. All procedures were approved by the Institutional Animal Care and Use Committee of the Pennington Biomedical Research Center and consistent with the NIH guidelines for the Care and Use of Laboratory Animals. Experiments were performed between April 2015 and March 2017. The mice were housed four per cage in plastic cages with bedding within a vivarium maintained on a 12 h light:12 h dark cycle, at a constant room temperature (22°C–24°C), and with free access to water and diet (AIN-93M diet Teklad, TD.94048.PWD).
Treatments
Quercetin glycosides (QG) were enzymatically manufactured at San-Ei-Gen F.F.I. (Osaka, Japan) from isoquercitrin prepared from quercetin-3-O-rutinoside and sophorin. Isoquercitrin was enzymatically modified with glycosyltransferase to add glucose residues. QGs were produced at >97% purity as measured by HPLC methods. This form of quercetin delivery was selected because QG are more water soluble and bioavailable than quercetin aglycone. When absorbed, QGs are enzymatically converted into the aglycone form and have beneficial effects similar to quercetin aglycone.
QG were delivered in the drinking water provided to the mice at two concentrations: 1.5 g/L (low) and 3.0 g/L (high) and control. Both concentrations had undergone pilot testing and found to be readily consumed by mice with no significant effects on water or food intake in the short term. Treatment was initiated at aged 24 weeks and continued until the mice were aged 48 weeks. Twelve mice were quasi-randomly assigned to the three treatment groups (balanced for starting body weights).
Measurements
Body composition: Body weight and composition were measured every 2 weeks during the study. Body weight was measured on an electronic scale, and body composition was measured using quantitative nuclear magnetic resonance (NMR; Minispec Mn10 NMR scanner; Brucker Canada, Milton, ON, Canada), as described previously [23].
Food intake: Amount of food intake was determined manually twice in individually housed mouse at 3 and 24 weeks of treatment. The daily food intake was determined over 3 days by the net reduction in diet weight with exclusion of the spilled diet.
Grip strength: The test was conducted on the fore limbs of each mouse using a grip strength meter (Columbus Instruments, Columbus, OH, USA) placed on the lab bench top. The experimenter held the mouse by its tail above the apparatus and allowed it to grasp with its front paws a horizontal metal bar. When the body of the mouse was parallel to the bench top, the experimenter provided a uniform pull on the mouse's tail away from the metal bar. The force produced during the pull on the bar was measured by the meter. The procedure was repeated three times at 5 s intervals for each mouse to obtain a mean value. The test was conducted two times—at 12 and 24 weeks following treatment.
Rotarod: An automated rotarod (Med Associates, St. Albans, VT, USA) was used as a behavioral test to assess motor function and coordination. The test included a slowly accelerating rod, progressing from 4 to 40 rpm during a period of 300 s, and the latency to fall was recorded for each mouse. Mice were given 3 trials per day, with an inter-trial interval of 60 min. Each trial lasted a maximum of 360 s. The mice were acclimated to the movement of the rod to remain on it for 90 s during the training session the day before the formal test. The test was performed two times in mice at 8 and at 20 weeks after the start of the treatment.
Treadmill test: After 16 weeks on treatment, the mice were trained to run on a motor-driven treadmill belt (Exer Gait XL, Columbus Instruments, Columbus, OH, USA) at a variable speed of 1.5– 12 m/min for 15 min. The training was performed during 2–4 sessions over a period of 1 week to make sure that all animals performed at their maximum. Parameters recorded included speed, time, and distance run.
Muscle morphology: At 48 weeks mice were euthanized by cervical dislocation and the carcass was quickly dissected to obtain muscle samples. Carefully dissected muscle samples included the gastrocnemius, the quadratus femoris, the tibialis anterior, and the soleus. Wet weights of the samples were then measured on an electronic balance. Muscle weights were then pooled for statistical comparison.
Statistical analysis: Statistical comparisons were made using unpaired t-tests. Statistical significance was accepted as p<0.05.
Body composition and food intake
As presented in Figure 1A-D, no significant effects of QG treatment were noted on overall body weight (A), lean mass (B), fat mass (C), or free fluid weight (D) as measured over the course of the study. A significant reduction in food intake was recorded in the low dose group after 3 weeks of treatment (Figure 2A), but this difference of about 1/3 gram had disappeared when intake was measured at 12 weeks into the study (Figure 2B). Thus, QG treatment had no overall long-term effects on body composition or food intake.
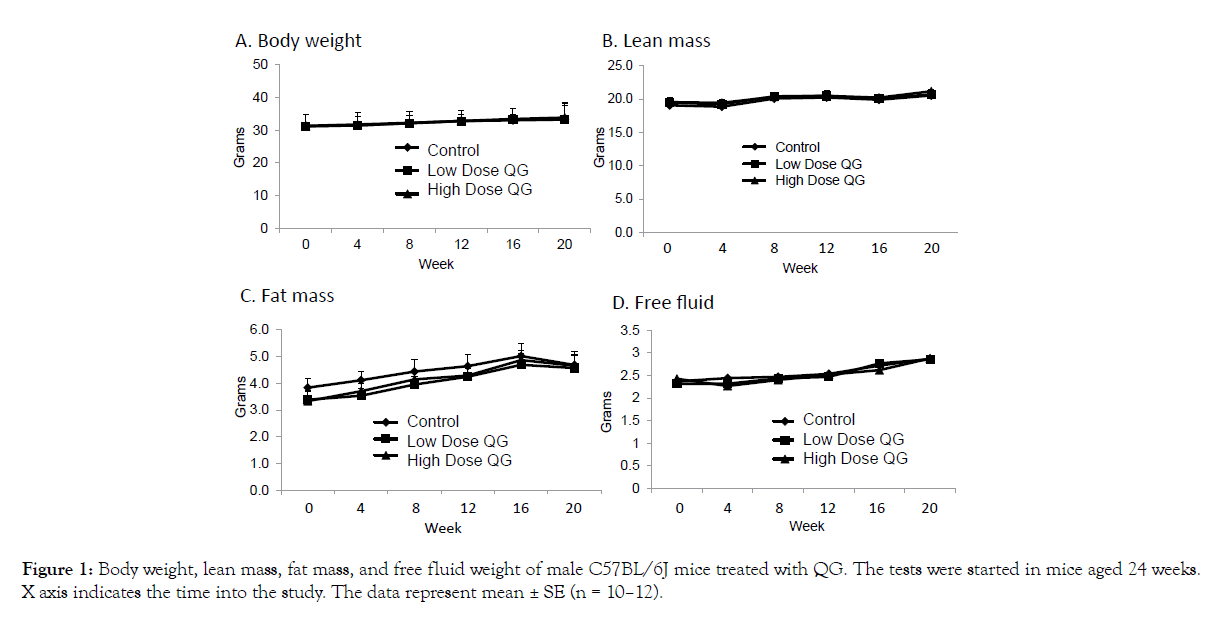
Figure 1: Body weight, lean mass, fat mass, and free fluid weight of male C57BL/6J mice treated with QG. The tests were started in mice aged 24 weeks. X axis indicates the time into the study. The data represent mean ± SE (n = 10–12).
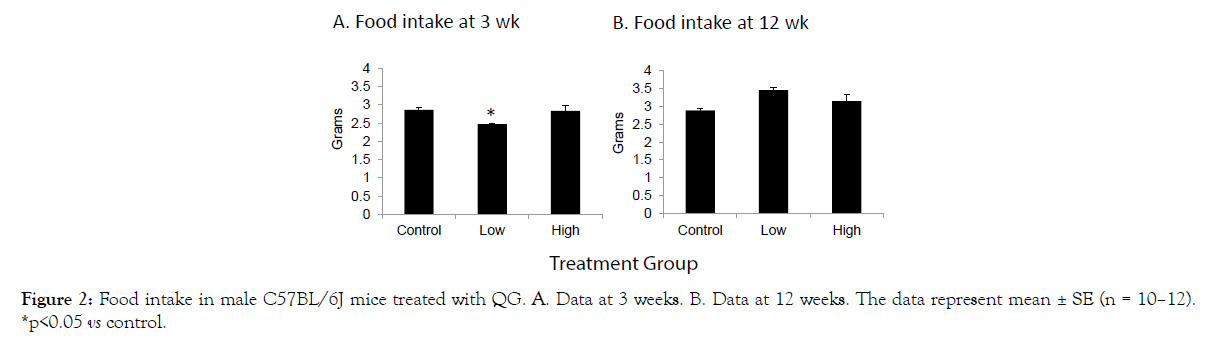
Figure 2: Food intake in male C57BL/6J mice treated with QG. A. Data at 3 weeks. B. Data at 12 weeks. The data represent mean ± SE (n = 10–12). *p<0.05 vs control.
Motor function
When examining the control groups only, as shown in Figure 3, a significant age-related decline was observed in the rotarod task but not in grip strength. QG supplementation markedly improved motor function in both tasks. At 12 weeks of treatment, grip strength was clearly increased at the low dose of QG and further improved at the high dose (Figure 3A). At 24 weeks, grip strength was significantly improved only in the high-dose group (Figure 3A). Similarly, rotarod performance was demonstrably and significantly improved in both dose groups, but the dose response was U-shaped (Figure 3B). At both 8 and 20 weeks, performance was most improved at the low dose, with a significant reduction in this effect at the high dose. In contrast, no significant treatment effects were observed in the measures of run distance, run speed, or run time assessed in the treadmill test at 16 weeks into the study (Figures 3C–E).
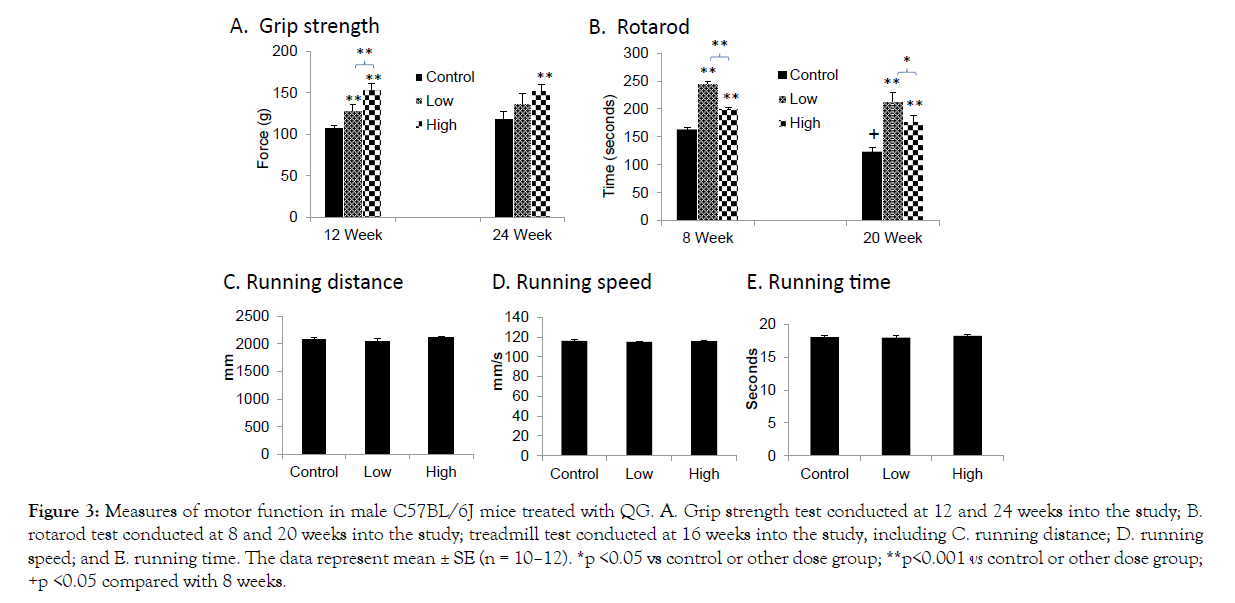
Figure 3: Measures of motor function in male C57BL/6J mice treated with QG. A. Grip strength test conducted at 12 and 24 weeks into the study; B. rotarod test conducted at 8 and 20 weeks into the study; treadmill test conducted at 16 weeks into the study, including C. running distance; D. running speed; and E. running time. The data represent mean ± SE (n = 10–12). *p <0.05 vs control or other dose group; **p<0.001 vs control or other dose group; +p <0.05 compared with 8 weeks.
Muscle morphology
After 24 weeks of QG treatment, a dose-related increase in the pooled weights of the muscle samples was observed, which was significant in the high-dose group (Figure 4).
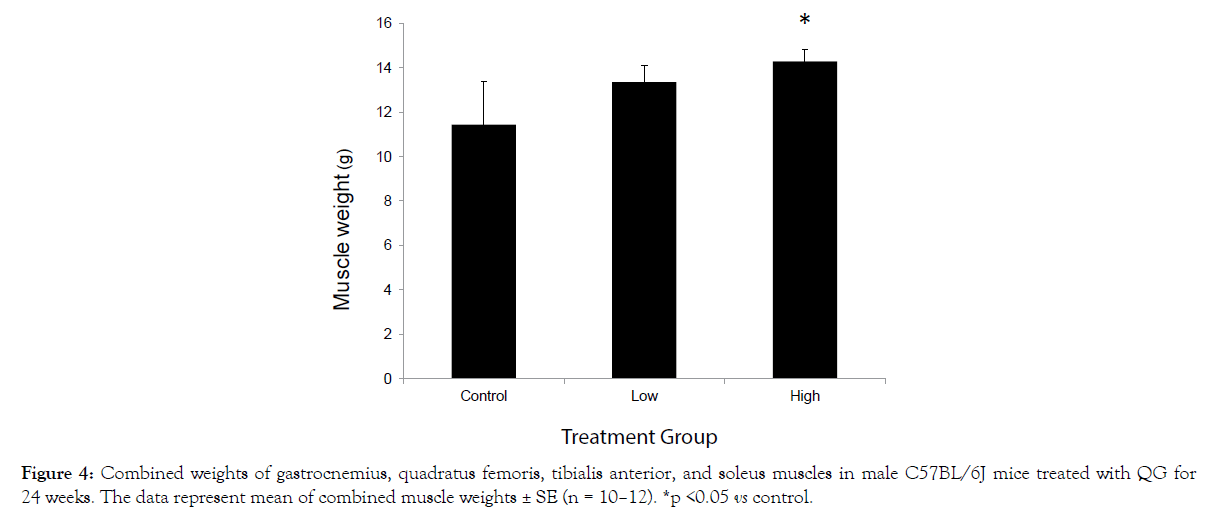
Figure 4: Combined weights of gastrocnemius, quadratus femoris, tibialis anterior, and soleus muscles in male C57BL/6J mice treated with QG for 24 weeks. The data represent mean of combined muscle weights ± SE (n = 10–12). *p <0.05 vs control.
Our results in this current study demonstrated that long-term treatment of adult mice with QG appeared to be safe and well tolerated in the two doses applied. Based on water consumption, the estimated daily doses were approximately 200 mg/kg as the low dose and 400 mg/kg as the high dose. No significant sustained effects were noted in body composition or food intake. These results generally agree with the literature noting the safety of quercetin consumption [24,25]. Food intake during quercetin treatment was not significantly reduced in mice on high-fat diets [26] or overweight humans [27]. However, mice receiving injections of quercetin (50 mg/kg) over 15 weeks showed significant body weight loss [28]. Henagan et al. [29] reporting on B6 mice on a high-fat diet fed a high dose of quercetin (600 μg/day) observed greater fat gains compared to the controls, whereas a low dose (50 μg/day) significantly reduced fat. Therefore, we conclude that the beneficial effects of oral quercetin intake on body composition are associated with high-fat diets in mice and not when QG is provided to mice on normal diets.
The most impressive findings were the marked improvements in grip strength and rotarod performance in QG-treated mice. Performance was improved by over 60% in both tests, with these improvements emerging after only 8 weeks of treatment. Aligned with these findings were the results showing that QG treatment increased muscle mass. We observed a significant dose-related increase in the absolute wet weight of the muscles examined; thus, this effect could explain the improvement in motor performance. The bulk of previous studies of quercetin in rodent models examining motor performance have employed models of defects rather than treatment of normal animals. As examples, various forms of quercetin supplementation in mice have attenuated the defects in motor function and morphology following chronic tail suspension [9], or denervation or crush of the sciatic nerve [10]. Likewise, quercetin treatment in murine genetic models of muscle dysfunction accompanied by motor impairments has also been effective [30]. Other models of brain injury, such as ischemia or delivery of neurotoxins, have been used to demonstrate beneficial protective effects of quercetin treatment on motor performance, including rotarod and grip strength [31-34]. In a recent study, Zhu et al. [35] found that the combination of quercetin and dasatinib acted as a senolytic drug—that is, one that promotes apoptosis of senescent cells. Specifically, they reported a reduction in the number of senescent cells in a progeroid mouse strain (Ercc1-/Δ) exposed to radiation. Treatment improved selected measures of health span in this short-lived mouse strain including treadmill performance and grip strength. This finding of improved motor performance was repeated recently in a study of aged mice treated with the combination of quercetin and dasatinib as well as a finding of increased survival [36]. Although this combination of compounds may provide some unique synergism, further investigation is necessary for confirmation of its efficacy, but the findings do further support the anti-aging effects of quercetin on motor performance. Regarding relevant findings of quercetin effects on muscle weight, a previous mouse study also reported increased muscle weights following quercetin supplementation, but this was found in mice on a high-fat diet that reduced muscle weights [11].
Noteworthy is that the doses of quercetin used in previous mouse studies have been generally much lower than those reported here in our study. Specifically, compared to estimated doses of 200 and 400 mg/kg provided orally in the current study, previous investigations have used doses typically under 100 mg/kg delivered via several routes, including intraperitoneal and via the nasal route. Chander et al. [32] reported that quercetin delivered orally in doses of 50 and 100 mg/kg could attenuate neurotoxicity induced by lead injections in mice.
The behavioral findings reported here appear unique regarding the beneficial effects of quercetin in normal mice at adult ages (<1 year). A previous study did report improved rotarod performance following quercetin treatment (orally for 5 days for 10–100 mg/ kg) in aged mice [2]. Behavioral aging—although the mice in the current study were not aged—was apparent in the results of the rotarod test, which confirmed observations from an investigation examining early signs of aging. Specifically, Shoji et al. [16] reported an age-related decline in rotarod performance of B6 mice aged from 2–12 months, but they did not observe a decline in grip strength across this age range, confirming our observation. Thus, the results emerging from our study are congruent with those of past studies showing functional improvements following quercetin treatment. While the current study was focused on the effects of QG on muscle and motor performance, our results should evoke further consideration of effects of QG supplementation on the brain. Relevant to this point, in recent studies of mouse models of Parkinson’s disease [37,38], treatment with epigallocatechin gallate, a phytochemical found in green tea, was reported to rescue deficits in rotarod performance and striatal dopamine levels induced by central injection of a neurotoxin (MPTP), whereas DL‑3‑n‑butylphthalide, a phytochemical extracted from grape seed, also rescued deficits in rotarod performance and midbrain dopamine neurons reduced by injections with an inflammatory toxin (LPS).
In contrast to the significant improvements in rotarod and grip strength performance, no significant treatment effects on performance on the treadmill were observed. The effects of quercetin on endurance have in previous studies yielded mixed results. For example, Lin et al. [39] reported that oral treatment of rats with quercetin-3-O-gentiobiose (25–75 mg/kg) could significantly improve the endurance capability of rats to fatigue in a swim test. Casuso et al. [40], however, found no significant effects of quercetin treatment in mice on endurance or VO2 max recorded on a treadmill. Several human studies conducted to examine the effects of quercetin supplementation on endurance and athletic performance have also yielded mixed results. In a recent meta-analysis of the literature, however, Somerville et al. [15] concluded that quercetin supplementation appears generally to improve athletic performance without negative effects. Although the reason why QG does not appear to improve performance only on the treadmill is not clear, differences in results on this parameter might be due to issues of dosage and duration of administration.
In summary, the results of our study support the conclusion that QG at the doses used (~200–400 mg/kg) can safely and effectively increase muscle mass that in turn translates into improved motor performance in adult mice. Specifically, QG significantly improved grip strength and rorarod performance after 12-week and 8-week treatment, respectively. QG also increased muscle mass, which is the likely mechanism for the improved motor performance. However, given the impressive effects of QG on motor function, future studies should focus on its effect on the brain. Additionally, future studies could focus on improving performance of aged individuals, particularly those showing signs of sarcopenia due to aging or other conditions, including obesity.
Citation: Kanzaki N, Takemoto D, Ono Y, Izumo T, Shibata H, Ye X, et al. (2019) Quercetin Glycosides Improve Motor Performance and Muscle Weight in Adult Mice. J Nutr Food Sci 9:760.
Received: 29-Apr-2019 Accepted: 28-May-2019 Published: 04-Jun-2019 , DOI: 10.35248/2155-9600.19.9.1000760
Copyright: © 2019 Kanzaki N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.