Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Review Article - (2023)Volume 13, Issue 2
The radon concentration in surrounding soil one of many parameters that impact on radon health risk of a house. The construction of the house, the porosity of the soil, the height of the ground water, and several other factors, are all-important. Even if there is no radon in the surrounding soil, the house may still be at risk if it has a well in the basement, or is built on rock, over a fissure. Nevertheless, it is often of interest to determine the radon concentration in soil gas. In this chapter, we focus on radioactive radon gas, its transmission methods of soil to the air, the most important health risks resulting from inhalation of smokers and non-smokers, colon and lung cancer resulting from it, the devices used in this year’s measurement. One of the most important devices used to measure its concentration are nuclear trace detectors. Solid state nuclear reagents are classified into two main categories: Organic reagents and inorganic reagents. Inorganic reagents are those reagents that do not include the elements carbon and hydrogen, and the most prominent types of these reagents that are widely used in the field of nuclear physics are mica and glass, and they are good inorganic reagents for recording the effects of neutrons and fission fragments and are used in detecting neutrons, especially in nuclear reactors, in order to withstand them high temperatures, and how to design trace detectors to measure the path of alpha particles for the purpose of measuring its.
Radon; CR-39; RAD-7; Polymer; Thoron; LR-115
Radon is one of the elements of the periodic table and falls within the group of noble elements (such as helium, neon, xenon, etc.) 9.7 kg/m3 and its boiling point -61.8ºC and its degree of freezing -71.0ºC. It is a colourless, odourless, radioactive gas, and these properties are difficult to detect. Radon is one of the heaviest known gases in nature. Its atomic number 86 mass number of its more stable counterpart is 222 [1]. Radon gas comes from the uranium decay chain 238 the found in the rocks of the earth’s crust, so the rate of radon flow varies from one region to another due to the difference in soil and rocks in the earth’s crust, which is the main source of uranium, and because the half-life of uranium is very long, the generation of radon remains continuous.
Radium is the main source of radon in nature and its halflife is equal to 1600 y, so it is expected to be present in all ores that contain 238 the uranium which are not distributed homogeneously in different geological areas due to the different geological composition of the components of the earth, so there are areas that are almost free of this element, while there are other areas that contain high concentrations of ores that contain this element, which significantly affects the concentrations of radon from one area to another. Because it is released naturally from land and groundwater into the atmosphere [2].
Isotopes of radon
In nature, there are four nuclear chains to which most of the radioactive elements belong namely uranium 238U and actinium 227Ac and thorium 232Th and neptunium 237Np each sequence begins with a nuclide or goes through a series of transformations that include the emission of alpha or beta particles to form a daughter nuclide. All the nuclei of isotopes in these chains are characterized by their atomic number that exceeds 82, which makes the electric forces of repulsion very strong inside the nucleus, meaning the instability of these elements and thus their dissolution, as each of these chains mentioned, except for neptunium because of its penetration from the globe, passes when dissolving one of the isotopes of radon. The three important ones are [3,4]:
Radon: It is an isotope of radon 222Rn which belongs to the uranium series 238U. This is the longest-lived of the isotopes of radon, as it has a half-life of (3.82 d), which increases its ability to spread over a few distances in the atmosphere, despite the fact that it is emitted from the soil in much smaller quantities than oxen.
Thoron: It is an isotope of radon 220Rn and belongs to the thorium series 232Th. It has a half-life (55 sec). Thoron is the most abundant isotope of radon due to the higher abundance of thorium compared to uranium, but it disappears from the atmosphere rapidly due to its short half-life.
Actinone: It is an isotope of radon 219Rn and belongs to the actinium series 227Ac. It has a half-life (4 sec) It is found in a very small percentage due to the lack of uranium 235U, and its half-life is short compared to uranium 238U [5].
Detection of radon
Solid state nuclear detectors: Because radon is a radioactive gas, the process of detecting it and measuring its concentrations in air, soil, water, etc., will be based mainly on the use of nuclear detectors. Here are many devices for detecting charged particles and they varied through the ionization caused by those particles in these detectors [6]. Among them are gaseous detectors, and they include three main types, which are the ionization chamber detector (Ionization chamber detector) and proportional detector (Proportional detector) and counter Kicker Muller (Giger-Muller dounter). The principle of work of these detectors is based on the ionization that occurs in the gas in the detector as a result of exposure to charged particles. Another device for detecting charged particles is the scintillation counter. Scintillation counter which works on the basis of converting the kinetic energy of particles into light flashes, depending on the properties of some organic or inorganic materials for the detector material that releases light flashes when charged particles fall on it. There are also semiconductor detectors (Semi-conductor detector (which is based on the principle of producing pairs of electrons and holes), electron-hole production (as a result of charge particles falling in the depletion area of these detectors, as in the surface barrier detector surface-barrier detector which is one of the good reagents for detecting alpha particles. Solid nuclear trace detectors have recently appeared SSNTD’s, they are electrically insulating solid materials to detect charged particles through damage (damage) left by those particles in these materials [7].
Solid nuclear detectors are defined as those solid insulating materials that have the ability to store the effect of ionizing radiation for a relatively long period of time and show them in the form of traces (T racks) of intense damage, as there is a noticeable change in the detectors in one or more of its features such as the diameter, length and density of the impact as a result of exposure to radiation [8].
The areas of damage can be seen either by using an electron microscope directly or by using an optical microscope after treating the reagents with some abrasive chemical solutions or by the plasma scraping process plasma etching. Abrasive chemical solutions attack the areas that have been exposed to radiation (damaged areas) at a greater rate than the healthy areas, because these areas are more fragile than the areas that were not exposed to radiation because they have a potential energy greater than the healthy areas as a result of the falling of charged particles on them, and thus their molecular weight decreases and the rate of attack increases chemicals for these damaged areas and thus leads to an increase in the rate of their decomposition (degradation) significantly. Solid nuclear trace detectors have appeared in the year 1958 which are noticed and worked at the atomic energy research foundation in Harwell in Britain that when you put a crystal of Lithium Fluoride(LiF). In contact with uranium flakes and exposing them to thermal neutrons, a number of effects appear after treatment with chemical solutions. Subsequently, studies in this field were carried out and then followed by collecting all those previous works and observations and entered them into the field of practical application [9]. This technology has been introduced into practical applications in general in 1977 after that, it developed and became an important branch of technological sciences and applications of this technology appeared in various fields is not confined to nuclear and radiological physics only, even if it is one of the most widely used fields, but it goes beyond that to biological, geological and chemical sciences. In view of the practical properties of solid nuclear trace detectors, in general such as ease of use, availability, low cost, and lack of need for a source of electrical energy, they have been widely used in many laboratories and by many researchers and in various applied fields. For example, its use in determining radon concentrations in the air and in soils and building materials [10].
Types of solid state nuclear detectors: Solid nuclear trace reagents are classified into two main categories: Organic reagents and inorganic reagents. Inorganic reagents are those reagents that do not contain the elements carbon and hydrogen, and the most prominent types of these reagents that are widely used in the field of nuclear physics are mica and glass, and they are good inorganic reagents for recording the effects of neutrons and fission fragments and are used in the detection of neutrons, especially in nuclear reactors, in order to withstand them high temperatures up to 400ºC [11].
As for the organic reagents, which include carbon and hydrogen in their composition, such as polymeric materials. Polymers are large molecules composed of small units linked together called monomers. Monomer consists monomer units in most of the plastics atoms linked together by covalent overcome hydrogen bond-carbon HC. In addition to carbon, hydrogen, oxygen and nitrogen atoms, organic polymers contain sulphur and halogens, knowing that most of the bonds that bind these atoms are easily broken when exposed to radiation [12].
One of the most prominent types of organic reagents is cellulosic reagents, and it includes several types, including cellulose nitrate CN-85 Cellulose acetate CA-80-15. These reagents contain nitrogen in their chemical composition. Cellulose nitrate detector CN-85 chemical composition is C6H18O5N2 and it is one of the best reagents for the detection of neutrons and charged particles such as protons and alpha particles and fission fragments and heavy ions as for the detector LR-115. It is one of the reagents for cellulose nitrate also its chemical structure is C12H7O16N3 and it is of several types according to its sensitivity to neutrons, namely:
LR-115 IB; LR-115 IIB; LR-115 I; LR-115 II. The detector is used LR-115II in calculating radon and uranium concentrations in soil, water, housing air, rooms and geological samples [13].
Polycarbonate reagents are also organic reagents that do not contain nitrogen, including the microfilm reagent. (Macrofol, Lexan and CR-39, PM-355). The chemical composition of macrofol reagent is C16H14O3. This detector has the advantage that it has two surfaces one smooth and the other rough and preferably a smooth surface facing the radioactive source and that the lack of distortions, which gives a clear impact. It is also one of the good reagents due to its high sensitivity to fission fragments and rapid neutron flux. It is used in the calibration of radon and the measurement of uranium concentrations. As for the Lexan reagent, its chemical composition is C16H14O3 [14]. It shares some chemical and physical properties with macrofol, and is one of the important reagents in detecting fission fragments, charged particles and heavy ions. The reagent after irradiation can be kept for a long time under different conditions of pressure and temperature. Promise CR-39 and the developed detector CR-39(DOP) and PM-355 It is one of the most important organic polycarbonate reagents because of its good detective and recording properties of charged particles [15].
The plastic nuclear detector CR-39 which was used in our current study has good specifications and characteristics that qualified it for use in many fields and applications. Hard plastic nuclear detector back CR-39 is a polycarbonate polymeric material which is abbreviated from Columbia resin, as it is one of the most common plastic reagents and is highly sensitive to charged particles. This reagent is prepared for the polymerization process of polyalyl dichlorocarbons (Poly allyl diglycol carbonate) and its partial form is nC12H18O7 and the percentage of hydrogen in it is in the limits 6.6% and its density 1.32 g cm-3 and its structural formula is represented in Figure 1 [16].
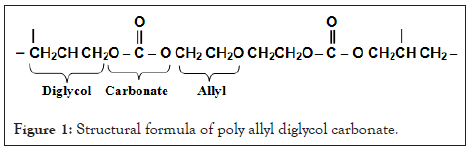
Figure 1: Structural formula of poly allyl diglycol carbonate.
This detector is characterized by its optical transparency, high sensitivity, homogeneity and regularity of its composition. The detector has been developed and improved through several experiments, as a detector was produced SR-86 developed from CR-39 and higher sensitivity. Another reagent form has also been produced CR-39 it is also developed from PM-355 [17], which is characterized by its high sensitivity to detect protons in particular prepare the detector CR-39. It is one of the solid reagents with high sensitivity to charged particles, as the main reason is attributed to the fact that this detector is a polymer with an organic composition that contains carbon bonds in its monomer, and these bonds are relatively weak and break easily when exposed to radiation [18]. To increase the sensitivity of this detector, weaker bonds than carbon bonds are introduced into its cross-linked lattice structure which made it more widespread and the best detector for recording the effects of nuclear particles, in addition to its other good specifications. The specifications and features of the nuclear trace detector can be summarized CR-39 and other nuclear detectors as follows [19]:
1. Its high sensitivity to radiation (alpha particles, protons and neutrons) and to different energies, as well as its ability to record the effects of rebound protons and for a wide range of energies, which makes it a sensitive detector for fast neutrons..
2. It has a transparency and purity of visual as well as the homogeneity of replay article (Homogenous). Its properties are similar (Isotropic).
3. Its resistance to moisture, heat and pressure during storage under normal conditions, in addition to its resistance to chemical solutions, except for strongly oxidizing bases.
4. It has a thermal stability (Thermoset) and it is characterized by its high thermal endurance and it cannot be converted from one form to another as a result of the intertwined structure of its chains, which determines the movement of the chains (cross linked) and partially crystallized, amorphous with ̴ 20% crystalline.
5. It is easy to use, its cost is low and it does not require complex electronic devices, as it has high engineering flexibility and it can also be prepared with dimensions commensurate with the working conditions, and the process of scraping the traces and showing them is an easy process.
6. Because of its ability to withstand atmospheric changes of temperature and humidity, it can be kept with most of the radioactive or scraped reagents for long periods of time, as it does not have a decay in the density of traces as a result of storage in normal weather conditions, except for storage at temperatures much higher than room temperature. As high temperatures work to fade and heal a number of effects formed in these substances, which leads to a decrease in their number.
7. High doses of rays (γ, X, UV) even electrons do not lead to the formation of traces, but they have important effects on the physical and chemical properties of the reagent material and in the factors of trace formation.
8. It has high sensitivity and efficiency, as some of these reagents have efficiency close to 100% such as mica and plastics, so it is used to measure the neutron flux or to measure neutron doses by measuring the effects left by the protons returning.
9. It does not dissolve in chemical solutions due to its high homogeneity and symmetry, but its thickness decreases by decomposition.
10. In addition to the characteristics mentioned above, it was found that the greater energy loss in the detector CR-39. The vertical fall of alpha particles occurs at 1.5 MeV approximately, the lowest energy that the detector can sense is about 200 KeV. Using ordinary chemical skimming, it represents the minimum threshold energy of the detector and100 KeV Approximately using electrochemical scraping Electro-Chemical Etching (ECE).
11. It was found that the detector CR-39 and its optical property changes as it get complete opacity and its colour tends to yellow when heated to a temperature 180ºC. It lasts for one hour and begins to decompose completely while being treated with a chemical scraper, which makes it impossible to obtain traces of the falling particles.
In view of the characteristics and features that the detector possesses CR-39 and mentioned above, it has been used in many applications and in many fields, including its use in the detection of protons, neutrons and alpha particles. It is also used in calculating radon concentrations in building materials, inside homes, buildings, soil [20].
RAD-7 solid state detector: The RAD-7 is a true, real-time continuous radon monitor. This means that a variable radon concentration level can be observed during a measurement period. This is very helpful, in the sense that one can investigate the factors influencing the radon concentration with time. The factors may include temperature changes, wind speeds, relative humidity and may even give insight into air movements in a room. Table 1 shows some properties of the detector [21].
| S.No | Specifications | Information |
|---|---|---|
| 1 | Modes of operation | Continuous radon gas monitoring |
| -Long-term/short-term screening | ||
| -Sniffer mode to search for radon and thoron entry points | ||
| -Grab mode protocols for radon in air and water | ||
| 2 | Measurements types | Measurement of radon in air, soil (with soil probe accessory) and water (with RAD H2O or RAD AQUA accessories) |
| 3 | Sensitivity | 0.0470 in (dps/150 Bq m-3) or 2.80 in (cpm/4 pCi/L) |
| 4 | Range | 0.1-10,000 pCi/L (4-400,000 Bq/m3) |
| 5 | Memory | 1,000 radon concentrations and associated data. Can be read out on LCD, downloaded to PC and/or printed out on HP IR printer. Summary of run shows high, low, average and standard deviation of readings. |
| 6 | Principle of operation | Electrostatic collection of alpha-emitters with spectral analysis |
| 7 | Power supply | AC or battery powered -5 AH 6V batteries; automatic battery charge when plugged in and switched on; optional low voltage input |
| 8 | Printer | Hewlett-Packard model HP 82240B |
| 9 | Dimensions | 24 cm x 19 cm x 27 cm |
| 10 | Weight | 5 kg |
Table 1: Properties of RAD-7 detector.
The RAD-7 radon detector manufactured by Durridge Company Inc. as shown in Figure 2 has been used for the radon and thoron concentration measurement in the soil, water and air samples [22].
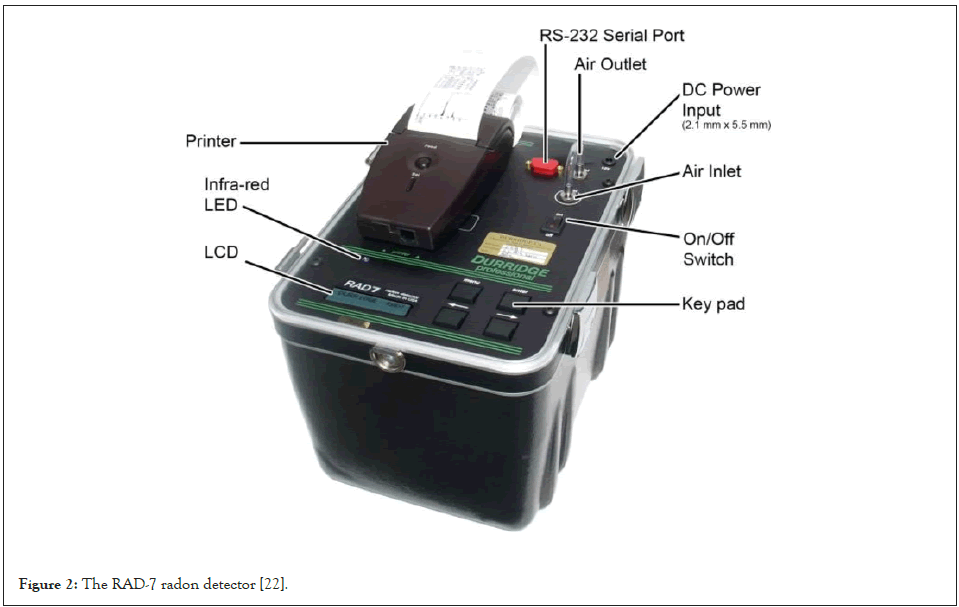
Figure 2: The RAD-7 radon detector [22].
The RAD-7 radon detector uses a solid state detector. This alpha detector is a silicon detector. The semiconductor material converts the alpha radiation from the decay of the radionuclide (e.g. 218Po or 214Po) into an electrical signal. One advantage of a solid state detector for radon or radon progeny detection is the fact that it can electronically determine the energy associated with the incoming alpha particle. In this way, the specific radionuclide can be identified, 218Po with an alpha radiation of 6.00 MeV or 214Po with an energy of 7.69 MeV [22].
The RAD-7 possesses an internal sample cell of about 0.7 liter and it has a hemispherical shape as it can be observed in Figure 2. The inside of the hemisphere is coated with an electrical conductor which can be changed with a high voltage power supply, to a potential of about 2000 V to 2500 V relative to the detector. This creates an electrical field throughout the cell. The electrical field propels the positively charged particles onto the detector in the periodic-fill cell. A decaying 222Rn atom within the cell leaves behind a positively charged 218Po, which is accelerated onto the detector and sticks to it. The 218Po nucleus has a relatively short half-life and when it decays, it will have a 50% chance of entering the detector where it will produce an electrical signal, and the energy of the alpha particle can be identified [23]. The electrical signal recorded from the decay of the radionuclide is then amplified, filtered and then sorted according to its strength. Different modes of functionality of the RAD-7 allow for detection of radon from the 218Po signal, but it can also determine the thoron (220Rn) concentration from the 216Po signal. The 218Po and 216Po signals arise from the 6.00 MeV and 6.78 MeV alpha decays respectively, and the alpha energies of the other decay products are ignored. Thoron is formed in the 232Th decay series see Table 1, subsequent nuclei from further decay include beta emitters, but the RAD-7 device is almost completely insensitive to beta decay. The RAD-7 determines the radon concentration by measuring the radioactivity of decay products [24].
The detector produces a spectrum that will be explained in the next subsection. A very important feature of the spectrum is the absence of the 5.49 MeV peak in the spectrum, since 222Rn decays in the air in the cell of the detector and not on the surface or close to the detector. The function of the desiccant in Figure 3 is to absorb any moisture that may be pumped into the tubing to keep the air relatively dry. However, the radon might also get adsorbed on the desiccant granules. This becomes a problem at very high radon concentrations, in which case the RAD-7 should be purged [24].
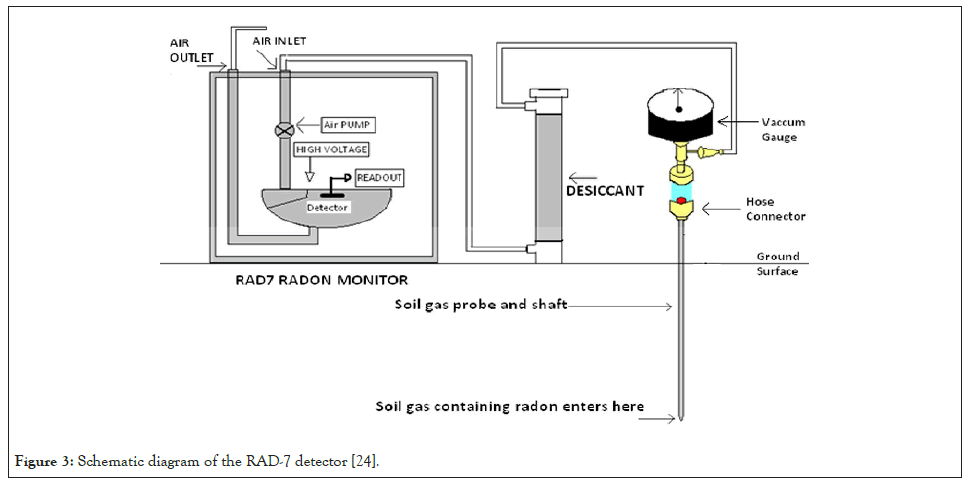
Figure 3: Schematic diagram of the RAD-7 detector [24].
RAD-7 spectrum analysis
The detector produces a signal with 50 % probability. This signal is intensified electronically and transformed into a digital signal. The microprocessor stores the energy level of the signal and produces the spectrum [25].
After the preparations of the measurement, we can pump radon containing air into the RAD-7. After a short time, we can see some counts in the energy interval A, which is the energy interval of the alpha decay of 218Po. Usually the counting rate increases in the first five minutes, because in this period of time the amount of positive ionized 218Po nuclei increases until it reaches a constant level on the detector. After 20 minutes the secular equilibrium state between 218Po and 222Rn is reached the activity of the daughter nucleus which is similar to the activity of the mother nucleus. At this time almost all counts can be found in the energy level at, which you can see in Figure 4. After a period of time we find that the counts per time in A are constant, but the overall counting rate increases. These new counts occur at the energy level B of the spectrum. They originate from the decay of 214Po which reaches its equilibrium state after 3 hours. In the full equilibrium state, the height of both peaks is almost equal, as shown in Figures 3 and 4 [26].
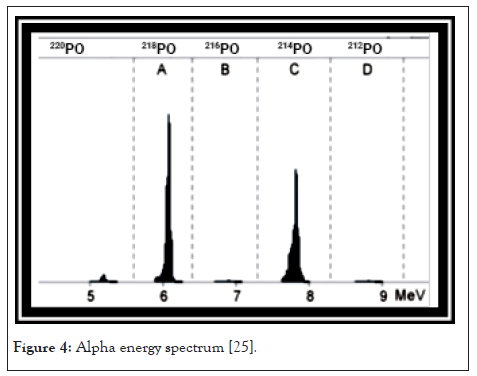
Figure 4: Alpha energy spectrum [25].
A spectrum is printed by the RAD-7 after the run that includes windows A-D. Windows E-H make up the composite window O. The different windows contain: (i) Window A: Total counts from 218Po decay. (ii) Window B: Total counts from 216Po decay. (iii) Window C: Total counts from 214Po decay. (iv) Window D: Total counts from 212Po decay. Window A and C are used to derive the radon concentration, while windows B and D account for thoron. The counts from the composite window are due to noise in the system.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Salman EF (2023) Radioactive Radon Gas and its Measurement Methods. J Phys Chem Biophys. 13:348.
Received: 22-Mar-2023, Manuscript No. JPCB-23-22320; Editor assigned: 24-Mar-2023, Pre QC No. JPCB-23-22320 (PQ); Reviewed: 07-Apr-2023, QC No. JPCB-23-22320; Revised: 14-Apr-2023, Manuscript No. JPCB-23-22320 (R); Published: 21-Apr-2023 , DOI: 10.35248/2161-0398.23.13.348
Copyright: ©2023 Salman EF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.