Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Review Article - (2017) Volume 7, Issue 3
Materials that undergo decay process to attain a stable configuration followed by the emission of particles such as alpha, beta and gamma are termed as radioactive elements. Radioactivity is measured in Curie (Ci) and Becquerel (Bq) SI units. Though radioactive elements are abundant on Earth and constantly decays to produce stable nuclei but such nuclear decay process may pose serious health threats to living beings. Therefore, must be regulated when working in vitro. Biosafety levels should be strictly observed when engaged in radioactive experiments to minimize contamination exposure.
<Keywords: Radioactive decay; Radioactive poisoning; Alpha particles; Beta particles; Half-life
In 1896 Henri Becquerel discovered phenomena of radioactivity by exposing potassium uranyl sulfate to sunlight, considering that it will emit neutral X-rays but in actual the emitted radiation was electrically charged since it bent in the magnetic field. So, the experiment was conducted using different elements that emits radiation that showed deflection in different directions in the magnetic field or not at all. Thus three different types of radioactive radiations i.e., negative, positive, and neutral were identified [1]. Marie Curie coined the term radioactivity and along with her husband Pierre, discovered other radioactive elements such as radium and polonium from radioactive ore of uranium for which she won a second Nobel Prize in 1911. Becquerel and the Curies received the Nobel Prize in 1903. The next important step down this road of discovery came from Ernest Rutherford who characterized the defining properties of these radioactive particles and named these as alpha, beta, and gamma particles. Classification of these radiations was based on their ability to penetrate matter. Alpha particles being massive moves slowly as compared to beta and gamma particles [2-4].
Radioactivity in nature
Elements with atomic number (Z) higher than Bi is radioactive in nature and are abundant in Earth. The earth also contains several elemental radioisotopes such as Potassium-40 which undergoes beta decays to form stable 40 Ar and 40 Ca. Uranium to lead ration revealed that Earth is approximately 4.5 billion years old. Rn 222, an intermediate product of U238 decay with 3.8 days half-life contributes in background radiation. Radioactive isotopes such as 7 Be and 14 C are constantly produced in the upper atmosphere. In order to gain understanding of Earth structural construct, study of radioactivity is crucial [5,6].
Radioactivity units
Becquerel (Bq) after Henri Becquerel is the unit in which radioactivity is measured and which the number of decays per second is.
One day per second equals one Becquerel
Curie another unit, named after Pierre and Marie Curie.
One curie is the activity of 1 gram of radium and equals 3.7 × 1010 Becquerel.
SI units and prefixes
Conference on Weights and Measures recommended the universal use of International System of Units for measuring radioactivity [7-9] (Tables 1 and 2).
| Radioactivity | Absorbed Dose | Dose Equivalent | Exposure | |
|---|---|---|---|---|
| Common Units | curie (Ci) | Rad | Rem | roentgen (R) |
| SI Units | becquerel (Bq) | gray (Gy) | sievert (Sv) | coulomb/kilogram (C/kg) |
Table 1: SI Units for measuring radioactivity.
| To convert from | To | Multiply by |
|---|---|---|
| Curies (Ci) | Becquerels (Bq) | 3.7 × 1010 |
| millicuries (mCi) | megabecquerels (MBq) | 37 |
| microcuries (µCi) | megabecquerels (MBq) | 0.037 |
| millirads (mrad) | milligrays (mGy) | 0.01 |
| millirems (mrem) | microsieverts (µSv) | 10 |
| milliroentgens (mR) | microcoulombs/kilogram (µC/kg) | 0.258 |
| Becquerels (Bq) | Curies (Ci) | 2.7 × 10-11 |
| megabecquerels (MBq) | millicuries (mCi) | 0.027 |
| megabecquerels (MBq) | microcuries (µCi) | 27 |
| milligrays (mGy) | millirads (mrad) | 100 |
| microsieverts (µSv) | millrems (mrem) | 0.1 |
| microcoulombs/kilogram (µC/kg) | milliroentgens (mR) | 3.88 |
Table 2: Conversion Equivalence.
Radiation measurements
Radiation measurements: Different related units are used for measuring radioactivity, absorbed dose, exposure and dose equivalent (r-e-a-d).
• Curie (Ci) and Becquerel (Bq) are the units for the measurements of radioactivity which refers to the concentration of emitted particles by a sample. It denotes the number of atoms in the given sample that decayed in a given time period.
• Coulomb/kilogram (C/kg) and Roentgen (R) are the units for exposure which is defined as the amount of radiation traveling through the air.
• Gray (Gy) and radiation absorbed dose (rad) measures the concentration of absorbed (deposited) radiation by a material or person.
• Sievert (Sv) and Roentgen equivalent man (rem) are units to measures dose equivalent also known as effective dose which is defined as the amount of absorbed radiation and its medical effects. Dose equivalent is same as the absorbed dose for beta and gamma radiation but larger than the absorbed dose for alpha and neutron radiation that are more damaging [10].
Types of radiations: Ionizing radiation emitted during radioactive decay are categorized as alpha, beta, gamma, protons, and neutrons.
Radioactive decay: Around 50 naturally occurring radioisotopes are found on earth. Impulsive radioactive fragmentation of an atomic nucleus to achieve a stable form by the emission of radiations is termed as radioactive decay [11]. Based on different types of radiations released during decay process there are three types of radioactive decays which are as follows:
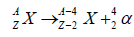
Alpha decay
Alpha particle (4He nucleus) is emitted during the decay process. Such nuclei have high proton to neutron ratio. Alpha particles are quiet stable in their configuration with two protons and two neutrons. Protons to neutrons ratio is reduced in the parent nucleus after decay by altering the Z. so the parent and the daughter atoms are chemically different elements [12].
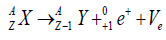
Beta decay
Beta particles are negatively charged electrons or positively charges electrons (positron) that is emitted from nuclei with too many protons during decay. There are two types of beta decays.
1) Beta minus decay: In which a neutron decays into a proton, an electron, and an antineutrino
2) Beta plus decay: In which a proton decays into a neutron, a positron, and a neutrino [13].
Gamma decay
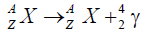
Through the emission of photons in gamma decay, the atomic mass of the parent and daughter nucleus remains the same. Thus parent and daughter atoms are chemically similar elements [14].
Half life: Half-life is defined as the time taken for one-half of the nuclei in a sample to decay. It is denoted by t1/2. Radioactive decay is as an exponential process. N denotes the number of original nuclei remaining after a time t from an original sample of N0 nuclei.
N=N0e-(t/T)
where T is the mean lifetime of the parent nuclei. Thus t1/2=0.693T [15,16]. Table 3 provides the half life of few radioactive elemts below.
| Radioisotope | Half-Life |
|---|---|
| Hydrogen-3 | 12.3 years |
| Carbon-14 | 5730 years |
| Phosphorus-32 | 14.3 days |
| Phosphorus-33 | 25.3 days |
| Sulfur-35 | 87.6 days |
| Iodine-125 | 60.1days |
Table 3: Half-life of raduioisotopes [17,18].
Properties of radioactive radiations
Alpha particles: Alpha particle contains two neutrons and two protons
• Particle carry positive charge.
• Mass of each alpha-particle is 4 times that of a proton or H-atom.
• Ionization power of alpha rays is very high.
• Penetration power of is very small
• Produce fluorescence in different substances
• Produce burn and source on human body
• Produce artificial radioactivity is certain nuclei
• They have strong ionizing power because they remove electrons from the atoms of gas through which they pass
• Their velocity range is 3 × 107 m/s to 3 × 106 m/s.
• Highly energetic emits 5 MeV.
• Travel only a few centimeters in air and is easily stopped by a paper sheer or the outer skin layer. Radon, uranium, radium, and thorium are alpha emitters..
Beta particles
• Negatively charged particles i.e., Electron
• Velocity is from 9 × 107 m/sec to 27 × 107 m/sec
• Affect the photo graphic plate
• Ionization power is very small
• Kinetic energy is less than that of alpha - rays.
• Produce fluorescence in different substance
• Produce secondary radiations called bremsstrahlung
• Travel several meters in air and millimeters into the human body and is stopped by an aluminum or plastic sheet. Sulfur-35, hydrogen-3 phosphorus-33, phosphorus-32 and carbon-14 are beta emitters.
Gamma particles
• Electrically neutral
• Travel with the velocity of light that is 3 × 108 m/sec
• Penetration power is very large. It is about hundred times larger than that of alpha rays.
• Produce feeble fluorescence
• Travel at the speed of light
• Could only be stopped by a thick sheet of lead, steel, concrete or several meters of water. Cobalt-60, cesium-137, zinc-65, and radium-22 are gamma emitters [19-22].
Radioactive poisoning
Numerous incidents in the past revealed that if biosafety levels are not strictly observed when working with radioactive isotopes, it increases the possibility of contamination with life threatening consequences. For example in 2006 murder of former Russian intelligence and death of Marie Curie’s daughter Irene by radioactive polonium. Similarly in 2003 a Russia journalist, Yuri Shchekochikhin and in 2004 a St Petersburg businessman Romam Tsepov died of radioactive poisoning [23]. Therefore it is important to keep in mind the safety considerations and measures while working in lab to ensure a radioactive free environment is established for protection of an individual from radioactive poisoning.
Safety considerations
Following safety levels should be observed while working with a radioactive material:
Defensive clothing: When working with an open radioactive source wear long length gloves and lab coats, closed toe shoes, safety glasses/goggles, radiation dosimeter, radiation monitor badges etc., to cover maximum parts of your body to avoid radioactive exposure because radioactive incidents frequently involves spills or splashes which can readily contaminate bare parts of the body.
Drinking/Eating: Drinking/eating, smoking etc., should be avoided where open radioactive sources present or stored to reduce the possibility of radioactive content intake in the body.
Oral pipetting: Mouth pipetting of radioactive solutions must not be avoided.
Security: Radioactive resources should be placed in shielded storage containers with proper labelling at secured places with maximum supervision and should not be left in an unattended lab or room.
Radioactive warning labels: Proper signs and symbols should be used to indicate the presence or storage of radioactive materials to avoid mishandling and exposure [24,25]. Labeling of rooms and containers of radioisotopes is mandatory under the following conditions [26,27] (Table 4).
| Radioactive isotopes | Activity (uCi) |
|---|---|
| H-3 | 1000 |
| C-14 | 100 |
| P-32 | 10 |
| P-33 | 100 |
| S-35 | 100 |
| Ca-45 | 100 |
| Cr-51 | 1000 |
| Fe-59 | 10 |
| Zn-65 | 10 |
| I-125 | 1 |
| U-238 | 100 |
Table 4: Radioisotope activity labelling.
Hoods and biosafety cabinets: Volatile radioelements i-125 or S-35 methionine/cysteine, must be handled inside specifically designed RAM hoods or safety cabinets equipped with alarming flow monitoring device to eliminate the risk of contamination.
ALARA: Radiation exposure must be kept as low as reasonably achievable means all safety measures and monitoring should be attempted to keep radiation doses ALARA [28].
Lab working concerns
• Be aware with the relative properties of the radioactive isotope that is to be used with any specific precautionary concerns specific to that radioisotope.
• Unexperienced nuclear procedures should be rehearsed before engaging with the actual radioactive substance following standard biosafety levels such as protective clothing, use of contamination free safety hoods, etc.
• Avoid direct handling of radioactive entities instead use remote handling tools and instruments.
• Proper storage of radioactive waste in specifically designed labelled storage cans with substantial Plexiglas shielding from external radiation levels.
• Authorized access to individuals to radioactive storage facilities with maximum security and supervision to avoid unauthorized access or breaching.
• Biosafety rules and regulations for working with radioisotopes should be made visible for everyone before the start of an experiment.
• Proper storage/disposal of radioactive waste in shielded cans and containers.
• Contamination monitoring should be done on regular basis after every nuclear experiment conducted.
• Washing sink for contaminated glassware and equipment should be clearly labelled with radiation warning signs [29,30].
Thus, radioisotopes are an abundant source of radioactive emissions that can be used in different biomedical applications but needs strict biosafety regulation to avoid exposure.