Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2022)Volume 10, Issue 8
Probiotics and prebiotics are known to benefit the host through modulating immune functions in multiple ways. In this study, selected probiotic lactic acid bacteria strains, various prebiotics, and synbiotic combinations of probiotics and prebiotics were investigated for anti-inflammatory effects in vitro models. Secreted Interleukin-8 (IL-8) from HT-29 cells was monitored as an inflammation parameter. Individually, the prebiotics raffinose, stachyose, verbascose, oligomate 55NP and beta-glucan decreased the secretion of IL-8 from stimulated HT-29 cells, as compared to stimulated HT-29 medium controls. With the exception of Bifidobacterium longum ATCC 15703, all the tested probiotic bacteria individually reduced secreted IL-8 levels. Synbiotic combinations of the tested probiotics with raffinose, soy extract or oligomate 55NP significantly reduced the IL-8 production in stimulated HT-29 cells, as compared probiotics alone. Several symbiotic combinations of stachyose, verbascose, Fructo-Oligosaccharides (FOS), inulin or beta-glucan with selected probiotic strains, decreased the secretion of IL-8. The combination of B. breve (FRP 334) with the Galacto-Oligosaccharides (GOS) type prebiotics, raffinose, stachyose, verbascose, oligomate 55NP, soy extract or galactobiose all significantly decreased the secretion of IL-8. Thus, this study demonstrated the ability of raffinose family oligosaccharides (raffinose, stachyose, verbascose) alone or in combination with probiotic bacteria to exert anti-inflammatory effects and thus modulate the immune system. In addition, the mixture of several oligosaccharides, such as soy extract, shows great ability to modulate immune function when combined with probiotics compared to the synbiotics of pure oligosaccharides with probiotics.
Raffinose; Probiotic; Prebiotic; Synbiotic; Interleukin-8; HT-29
The effects of probiotic bacteria and prebiotics on the host, particularly on the inflammation and immunomodulation of the host, have been extensively investigated. Probiotics are living microorganisms that exert beneficial effects on the health of the host when administrated in adequate amounts (FAO/WHO, 2002). Research on probiotic bacteria demonstrated a benefit to the host through stimulating lymphocyte proliferation [1], enhancing innate and adaptive immunity [2], inhibiting pathogen adherence and virulence-related gene expression [3,4], and preventing apoptosis of intestinal epithelial cells [5]. In particular, lactic acid bacteria, such as Bifidobacterium and Lactobacillus, have been shown to have therapeutic properties associated with Inflammatory Bowel Disease (IBD) [6,7]. Prebiotics are selectively fermented food ingredients that stimulate specific changes, both in the composition and/or activity of the gastrointestinal microflora, which in turn, confers benefits on the host’s well-being and health [8]. Previous studies have demonstrated that diets enriched with prebiotics increase the number of probiotic bacteria, and/or their fermentation products in the colon [9]. Short-chain fatty acids, the predominant fermentation products, have been associated with calcium, magnesium and water absorption [10,11]. Furthermore, it has been suggested that prebiotics can benefit the host directly through providing energy to the colonic epithelium, increasing epithelial cell growth [12] and reducing pathogenic bacteria adhesion and invasion [13,14].
Interleukin-8 (IL-8) is a low molecular weight cytokine with potent chemotactic properties and serves to amplify the inflammatory cascade [15,16], which plays an important role in Inflammatory Bowel Disease (IBD). Upon stimulation with stimuli such as Tumour Necrosis Factor (TNF) and Interleukin-1 β (IL-1 β), mononuclear cells, endothelial cells, and epithelial cells can secrete IL-8 [17]. Thus, secreted IL-8 can be monitored as an inflammatory biomarker. Previous researchers have demonstrated that some probiotic bacteria, particularly bifidobacteia and lactobacilli, showed high levels of inhibition of IL-8 release [18,19], thereby indicating potential anti- inflammation effects. Recent studies on the prebiotics Fructo-Oligosaccharides (FOS) and Galacto-Oligosaccharides (GOS) have shown anti-inflammatory effects and the mechanisms underlying are currently believed to be an indirect effect on the growth and metabolism of probiotic bacteria [20]. These observations have led to the development of the concept of synbiotics where prebiotics and probiotics are used in combination, exerting an increased benefit to the host, as compared to the use of either the probiotic or prebiotic alone [21,22]. With a decreased reliance on antibiotics for disease control, the use of synbiotics has become a popular alternative presently being investigated within the research community, including the administration of microencapsulated synbiotics as food additives in broiler chickens prior to slaughter to reduce the presence of C. jejuni in the poultry food chain [23].
The HT-29 colonic epithelial cells secrete IL-8 and other cytokines in response to invasive bacteria or stimuli and therefore, represent a model used to evaluate the immune function of intestinal epithelial cells [24,25]. The aim of this study was to investigate the anti-inflammatory characteristics of potential synbiotic combinations in vitro, by assessing levels of the inflammatory biomarker, IL-8. Results from this study will direct further investigations of the benefits of synbiotics in animal models.
Bacterial strains and cell culture conditions
The bacterial strains used in this study, which were isolated from farm animals, dairy and human sources, are listed in Table 1. Lactobacillus species and Pediococcus species were grown in a 5% (v/v) CO2 atmosphere at 37°C in modified Mann-Rogosa-Sharpe broth (mMRS), Difco; 0.2 g sodium carbonate, 0.1 g calcium chloride in 1 L distilled water and 5% (w/v) filter sterilized L-cysteine HCl monohydrate (FisherBiotech) for 17 hours. Bifidobacterium species were grown in Reinforced Clostridial Medium (RCM) broth (Oxoid) at 37°C for 48 hours under anaerobic atmosphere using GasPakTM EZ Anaerobe Container System Sachets (ACSS) (Becton Dickinson and Company, Maryland, USA) in the GasPakTM EZ incubation container (Becton Dickinson and Company). Dry anaerobic indicator strips (Becton Dickinson and Company) were used to verify that an anaerobic atmosphere was maintained (Table 1).
| Strains | ATCC or FRPc | Source of isolation | Concentration (OD600) |
|---|---|---|---|
| Lactobacillusspecies | |||
| L. rhamnosus GG | ATCC 53103 | Human feces | 0.101 ± 0.01 |
| Bifidobacteriumspecies | |||
| B. adolescentis | ATCC 15703 | Intestine of adult | 0.661 ± 0.01 |
| B. longum | ATCC 15708 | Intestine of adult | 0.458 ± 0.01 |
| B. breve | FRP 334 | Human infant | 0.456 ± 0.01 |
| Pediococcusspecies | |||
| P. acidilactici | FRP 236 | Meat-associated | 0.245 ± 0.01 |
| P. pentosaceus | FRP 243 | German sausage | 0.257 ± 0.01 |
| P. pentosaceus | FRP 244 | German sausage | 0.148 ± 0.01 |
Note: ATCC: American Type Culture Collection; FRP: Guelph Food Research Centre (GFRC) culture collection of Agriculture and Agri-Food Canada
Table 1: Probiotic strains used in the study.
The human colonic epithelial cell line HT-29 (American Type Culture Collection, Rockville, MD, USA) was cultured in a 75 cm2 flask (Corning, New York, USA) with McCoy’s 5A medium (Invitrogen, Ontario, Canada) supplemented with 10% (v/v) Fetal Bovine Serum (FBS) and antibiotic-antimycotic solution (10000 U/ml penicillin G, 10 mg/ml streptomycin and 25 μg/ml amphotericin B; Sigma-Aldrich). HT-29 cells monolayers were maintained in 5% CO2-95% air in a humidified atmosphere at 37°C and were sub-cultured using 0.25% (v/v) trypsin-Ethylene Diamine Tetra Acetic acid (trypsin-EDTA) prior to reaching confluence.
Prebiotics
Nine different dietary fibers were tested. Table 2 shows the carbohydrates, sources, concentration and the commercial sources (Table 2).
| Substrate | Source | Manufacturer | Concentration (mg/ml) |
|---|---|---|---|
| Fructooligosaccharides (FOS) | Chicory | Sigma | 10 |
| Inulin | Chicory | Sigma | 10 |
| β -glucan (low viscosity) | Barley | Megazyme | 10 |
| Galactooligosaccharides (GOS) | Soy | GFRC | 10 |
| Oligomate 55NP | Milk | Yakult, Tokyo, Japan | 10 |
| D-(+)-Galactose | Chicory | Sigma | 10 |
| Galactobiose | Galactan | Megazyme | 10 |
| Raffinose | Stachys tuberifera | Sigma | 10 |
| Stachyose | Sigma | 10 | |
| Verbascose | Pea molasses | Megazyme | 10 |
Note: GFRC: Guelph Food Research Centre of Agriculture and Agri-Food Canada
Infection assay
At the beginning of each experiment, HT-29 cells were seeded at 5 × 105 cells/ml/well in 10-well cell culture plates and grown at 37°C under 5% CO2 atmosphere to reach confluence (about 3 days). The cells were washed with Phosphate Buffered Saline (PBS), pH 7.4 to remove culture medium and non- adherent cells. Fresh antibiotic-free McCoy’s 5A media containing prebiotics were added to each well. Selected probiotics were grown as previously described, washed three times in PBS and re-suspended in complete McCoy’s 5A media (antibiotic-free) and the Optical Density (OD600) of each strain was adjusted to the ones indicated in Table 1. Probiotics were co-incubated with HT-29 cells at 37°C for 3 hours. Interleukin-8 (IL-8) production was assessed by adding interleukin-1 β (IL-1 β) to a final concentration of 10 ng/ml. Following 4 hr incubation at 37°C, the culture supernatants were collected, centrifuged and stored at -20°C for future analysis. HT-29 cells treated only with IL-1 β were used as positive controls. Cell cultures without probiotic bacteria in the culture medium and medium containing prebiotics, and the co-cultures of probiotics and HT-29 cells in culture medium lacking prebiotics, were used as controls.
Cell viability was verified by a Trypan blue exclusion assay after collecting the supernatant. HT-29 cells viability under the described experimental conditions was greater than 95%.
Measurement of interleukin-8
The amount of extra-cellular IL-8 protein secreted from HT-29 cells was determined by a sandwich enzyme immunoassay using the commercially available Quantikine® Human CXCL8/IL-8 kit. The assay was performed at room temperature according to the manufacturer’s instructions using thawed samples. The supplied monoclonal antibody was specific for IL-8.
Prior to Enzyme Linked Immunosorbent Assay (ELISA) analysis, supernatants were diluted 10-fold with the supplied calibrator diluent RD5P. Following the ELISA procedure, sample absorbance values were read immediately in duplicate at 450 nm on a BioTek PowerWave XS Universal Microplate Spectrophotometer (Vermont, USA). A correction wavelength of 570 nm was used. IL-8 concentrations were calculated from the straight-line portion of a standard curve using duplicate readings of the recombinant human IL-8 control supplied with the CXCL8/IL-8 kit. According to the manufacturer, the mean minimum detectable dose of IL-8 was 3.5 pg/ml. The concentrations of IL-8 showed in the figures were multiplied by the same dilution factor (10-fold).
Statistical analysis
Each experimental condition was investigated by three independent trials. ELISA 152 absorbance readings were conducted in duplicate for each sample. Statistical significance 153 between treatment and control conditions was assessed by the one way Analysis of variance (ANOVA) (IBM 154 SPSS versions 16.0). P values of <0.05 were considered statistically significant.
With the exception of galactobiose and soy extract, none of the remaining prebiotics induced secretion of IL-8 when they were co-culture with unstimulated HT-29 cells. Upon stimulation with IL-1 β, HT-29 cells alone and in combination with all the prebiotics tested increased IL-8 levels.
The effect of synbiotics of GFO family with probiotics on the production of interleukin-8
The combinations of raffinose or oligomate 55NP with all the selected probiotics significantly (P<0.05) reduced the secretion of IL-8 from stimulated HT-29 cells, as compared to probiotic alone. Five strains, L. rhamnosus GG (ATCC 53103), B. adolescentis (ATCC 15703), B. longum (15708), B. breve (FRP 334) and P. pentosaceus (FRP 243) combined with stachyose significantly (P<0.05) decreased the production of IL-8 from stimulated HT-29 cells. The synbiotics combinations of verbascose with B. breve (FRP 334) and P. pentosaceus (FRP 244) also significantly reduced (P<0.05) the secretion of IL-8 in stimulated HT-29 cells (Figures 1a-1d).
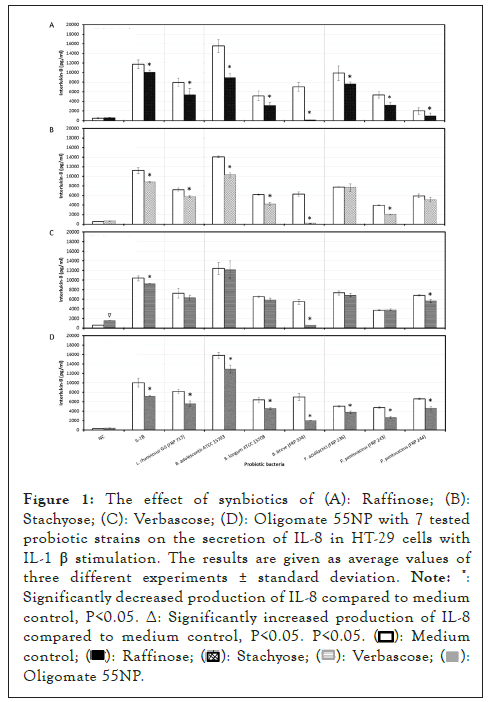
Figure 1: The effect of synbiotics of (A): Raffinose; (B):
Stachyose; (C): Verbascose; (D): Oligomate 55NP with 7 tested
probiotic strains on the secretion of IL-8 in HT-29 cells with
IL-1 β stimulation. The results are given as average values of
three different experiments ± standard deviation.
The influence of galactose and galactobiose on the production of interleukin-8 when combined with probiotics
Galactose alone did not show any effect on reducing secreted IL-8 in stimulated HT-29 cells. With exception of the synbiotic combinations of B. breve (FRP334) with galactose, the remaining synbiotics did not significantly reduce the secretion of IL-8 from stimulated HT-29 cells, as compared to probiotics alone. Upon the addition of galactobiose to stimulated and unstimulated HT-29 cells, IL-8 secretion levels increased significantly (P<0.05). The combinations of galactobiose with tested probiotic bacteria, with the exception of B. longum (ATCC 15708) and B. breve (FRP 334), all increased the secretion of IL-8 from stimulated HT-29 cells, as compared to probiotics controls (Figures 2a and 2b).
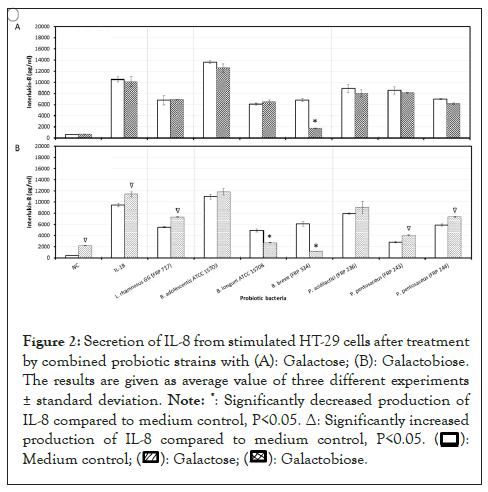
Figure 2: Secretion of IL-8 from stimulated HT-29 cells after treatment
by combined probiotic strains with (A): Galactose; (B): Galactobiose.
The results are given as average value of three different experiments
± standard deviation.


The effect of synbiotics of FOS-type prebiotics with probiotics on the production of interleukin-8
In combination with FOS, B. longum (ATCC 15708), B. breve (FRP 334), P. acidilactici (FRP 236) P. pentosaceus (FRP 244 and FRP 243) all significantly (P<0.05) decreased IL-8 production from stimulated HT-29 cells. In contrast, only B. longum (ATCC 15708) combined with inulin from chicory significantly (P<0.05) reduced the secretion of IL-8 in stimulated HT-29 cells, as compared to probiotics alone (Figures 3a and 3b).
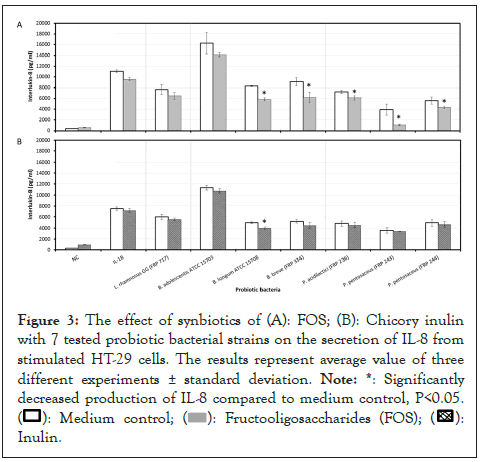
Figure 3: The effect of synbiotics of (A): FOS; (B): Chicory inulin
with 7 tested probiotic bacterial strains on the secretion of IL-8 from
stimulated HT-29 cells. The results represent average value of three
different experiments ± standard deviation.
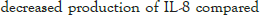
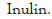
The effect of synbiotics of β -glucan with probiotics on the production of interleukin-8
As illustrated, although all the synbiotic combinations of β -glucan (from barley) with selected probiotics reduced IL-8, a significant reduction (P<0.05) was observed with the probiotic strain B. breve (FRP 334), and P. pentosaceus (FRP 243 and FRP 244) (Figure 4).
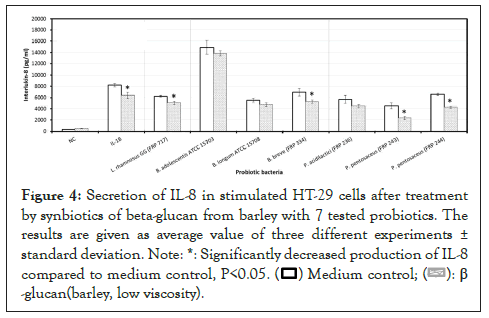
Figure 4: Secretion of IL-8 in stimulated HT-29 cells after treatment
by synbiotics of beta-glucan from barley with 7 tested probiotics. The
results are given as average value of three different experiments ±
standard deviation.
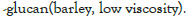
The effect of soy extract and its combinations with probiotics on the production of interleukin-8
For both stimulated and unstimulated HT-29 cells, soy extract increased IL-8 levels in HT-29 cells. Whereas, all seven combinations of soy extract with probiotic bacteria significantly (P<0.05) decreased the production of IL-8 in stimulated HT-29 cells (Figure 5).
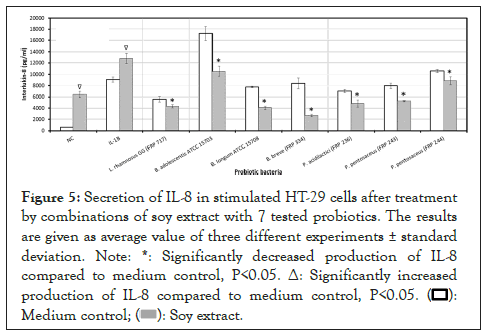
Figure 5: Secretion of IL-8 in stimulated HT-29 cells after treatment
by combinations of soy extract with 7 tested probiotics. The results
are given as average value of three different experiments ± standard
deviation.

The effect of combinations of bifidobacteria with various prebiotics on the production of interleukin-8
The synbiotic combinations of B. breve (FRP 334) with various prebiotics. Results indicated raffinose, stachyose, verbascose, galactose, galactobiose, Oligomate 55NP and soy extract significantly reduced the secretion of IL-8 (P<0.05) when stimulated HT-29 cells were co-incubated with B. breve (FRP 334). Additionally, the production of IL-8 from the HT-29 stimulated cells was significantly reduced in the presence B. breve (FRP 334) combined with β -glucan, as compared to B. breve alone (Figure 6).
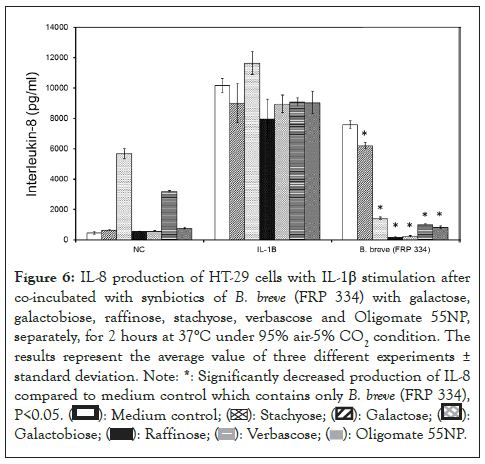
Figure 6: IL-8 production of HT-29 cells with IL-1β stimulation after
co-incubated with synbiotics of B. breve (FRP 334) with galactose,
galactobiose, raffinose, stachyose, verbascose and Oligomate 55NP,
separately, for 2 hours at 37°C under 95% air-5% CO2 condition. The
results represent the average value of three different experiments ±
standard deviation.

Compared to IL-1 β stimulated HT-29 controls, B. adolescentis (ATCC 15703) increased the production of IL-8 in HT-29 cells, however, the combinations of B. adolescentis with raffinose, stachyose, oligomate 55NP or soy extract showed a significant (P<0.05) decrease in secreted IL-8, as compared to B. adolescentis alone.
Recent research on probiotics and prebiotics has demonstrated anti-inflammatory properties, and with the development of synbiotic combinations, the increased benefits to human health and possibilities of use in functional foods will be immense. In this study, the anti-inflammatory characteristics of potential synbiotic combinations were evaluated in vitro, by assessing levels of the inflammatory biomarker, IL-8. As both galactobiose and soy extract demonstrated intrinsic pro-inflammatory characteristics in unstimulated HT-29 cells, their use as a prebiotic alone may not be suitable for human applications. With respect to stimulated HT-29 cells, we demonstrated that the remaining prebiotics elicited differing anti-inflammatory properties with respect to IL-8 levels, which varied according the selected probiotic in synbiotic combination.
Galacto-oligosaccharides
Our previous study demonstrated that prebiotics with linear chains consisting of galactose units (Galacto-Oligosaccharides (GOS)) were better utilized by probiotics compared to those consisting of glucose and fructose units [26]. In the present study, the synbiotic immunological effects on IL-8 production in stimulated HT-29 cells in the presence of prebiotics, including raffinose, stachyose, verbascose, oligomate 55NP, soy extract, and galactobiose, with the seven probiotic strains were evaluated. Results indicate that most synbiotic combinations of tested probiotic bacteria with GOS-based prebiotics significantly reduced the production of IL-8. Particularly, the combinations of B. breve (FRP 334) with all the GOS members significantly decreased the IL-8 secretion in HT-29 stimulated cells. In a recent study by Grimoud et al. an anti-inflammatory effect and anti-proliferative characteristics were observed for synbiotic combination of B. breve R0070 and L. lactis R1058 with oligoalternan, which is a GOS. A dose range assay demonstrated that both B. breve R0070 and L. lactis with oligoalternan were required to elicit the anti-proliferative effects in a dose dependent manner. The authors concluded that the anti-proliferative effect was likely a result of the additional effects of the mixture of probiotic strains and the intrinsic effect of prebiotics. Further, Garrido et al. illustrated phenotypic differences in GOS consumption among 22 isolates of B. longum subspecies infantis, which may be attributed to the variable expression of β -galactosidases in the presence of GOS.
Raffinose Family Oligosaccharides (RFO)
Raffinose, stachyose and verbascose belong to the Raffinose Family Oligosaccharides (RFOs), which occurs mainly in plant seeds and can be classified as α -GOS [27-29]. Although raffinose has long been regarded as a non-digestible oligosaccharide, research indicates a beneficial effect of raffinose oligosaccharides on the gut microflora [30]. Sonoyama et al. investigated the effect of raffinose on the immune system and demonstrated that dietary raffinose suppressed allergic airway eosinophilia in ovalbumin-sensitized Brown Norway rats, which suggests that dietary raffinose affects immune health to prevent atopic asthma. The encapsulation of B. breve JCM1192T with raffinose was also shown to improve epithelial proliferation in the rat small intestine, whereas encapsulated B. breve alone was not detected in cecal rat contents [31]. The present study provides the first evidence that RFOs contribute to immunomodulation through reducing the pro-inflammatory cytokine IL-8 in stimulated colonic epithelia cells HT-29 in an indirect way. RFOs synbiotic combinations reduced IL-8 levels, whereby combinations of raffinose or stachyose with B. breve (FRP 334), B. adolescentis (ATCC 15703), and P. pentosaceus (FRP 243) provided the greatest decrease in IL-8 secretion. Similarly, the synbiotic combination of verbascose with B. breve (FRP 334) significantly decreased the secretion of IL-8 from HT-29 cells. These results demonstrate that synbiotics of RFOs with probiotics have potential immunomodulatory effects in vitro. Our previous study demonstrated that GOS-type oligosaccharides all promoted the growth of the same tested probiotic bacteria. In the present study, the synbiotics of raffinose and oligomate 55NP with all 7 bacterial strains reduced the secretion of IL-8 from stimulated HT-29 cells, however, only a few synbiotics of stachyose and verbascose with these bacterial strains showed anti-inflammatory effects. These results suggest that in addition to improved bacterial growth by GOS-type oligosaccharide substrates, unknown mechanisms may also be involved with the immunomodulatory effects of the synbiotics. Compared to stimulated HT-29 cell controls, RFOs and oligomate 55NP independently reduced secreted IL-8, indicating that these prebiotics alone have immunomodulatory properties. Eiwegger et al. reported that epithelial cells can transfer prebiotic oligosaccharides depending on the molecular size and structure; this may be one of the mechanisms underlying the immunomodulatory effects of these prebiotics alone.
Galactose is the chain component of GOS and improved the growth of all 7 tested probiotic strains. With the exception of the synbiotic combination of galactose with B. breve (FRP 334), the remaining synbiotic combinations with galactose did not significantly reduce secretion of IL-8. With respect to the synbiotic combination of probiotics with galactobiose, which is a disaccharide of galactose, B. longum (ATCC 15708) and B. breve (FRP 334) significantly reduced IL-8 secretion, compared to galactobiose or probiotics alone. Furthermore, while the abilities of synbiotics of B. breve (FRP 334) with GOS-type oligosaccharides and galactose to decrease IL-8 secretion differ, only minimal amounts of IL-8 was produced after two-hour co-incubation of HT-29 cells with the combinations of raffinose, stachyose, verbascose and oligomate 55NP with B. breve (FRP 334). In contrast, the production of IL-8 from HT-29 cells treated with galactose and B. breve (FRP 334) was still elevated. This may be due to the preferential consumption of oligosaccharides by probiotic strains, which can be an advantage for the strains competing with other microorganisms in the human gut for nutrition, where oligosaccharides are the primary sugars.
Soy extracts (GOS-type oligosaccharides)
The main components of soy extract are stachyose (10.51%, w/w), galactose (4.11%) and fructose (4.26%). The raffinose and verbascose content was 1.18% and 0.55% respectively. When combined soy extract, all tested probiotic bacteria significantly reduced the production of IL-8 in stimulated HT-29 cells, though soy extract independently and in combination with IL-1 β stimulated IL-8 secretion. Currently, there are no other studies which have investigated the use of soy extract in synbiotic preparations. The results obtained in our study indicate that the composition of soy extract, containing several RFOs, elicited an immunomodulatory response when combined with probiotic bacteria, as compared to the individual soy extract components combined with probiotic strains.
Fructo-Oligosaccharides (FOS)/inulin
The immunomodulatory effects of synbiotics of inulin and Fructo-Oligosaccharides (FOS) with 7 probiotic strains was also tested. The majority of FOS synbiotics with tested probiotic bacteria decreased the production of IL-8 in HT-29 cells, whereas, only synbiotics of B. longum (ATCC 15708) with inulin demonstrated effects on reducing secretion of IL-8 in HT-29 cells, compared to probiotics alone. Presently, research conducted on the immunomodulatory effects of FOS or inulin as components of synbiotics have been limited to in vivo studies. Seifert and Watzl demonstrated that the synbiotics of FOS/inulin with probiotics in humans and animals had a strong impact on the immune system [32]. Roller et al. indicated that consumption of FOS-enriched inulin in combination with probiotics L. rhamnosus GG and B. lactis had minor stimulatory effects on polypectomised and colon cancer patients. In a similar study by Rafter et al. a synergistic change in cancer biomarkers was also observed in polypectomized and colorectal cancer patients following treatment with B. lactis BB10 and L. rhamnosus GG with oligofructose-enriched inulin. Varying results from different researches may be due to the variety of sources and administration methods of prebiotics, as well as the possibility that synbiotics preferentially affect some parts of immune systems, immune parameters or organs.
β -glucan
The immunomodulatory effects of β -glucans have been well studied and in vitro assays have demonstrated that β -glucans enhanced the innate immune response of leukocytes and epithelial cells, primarily by modulating the cytokine production [33,34], whereby pro-inflammatory cytokines (tumor necrosis factor alpha, IL-6, IL-8) and anti-inflammatory cytokines (IL-10) were increased [35,36]. β -glucans, which are carbohydrates consisting of glucose polymers, represents major structural components of yeast, fungi, and bacteria cell walls. Present in the endosperm cell wall of cereals, such as barley and oat, β -glucan is also present in our daily diet, suggesting that immune modulation may be possible by increasing dietary β -glucan intake, such as with functional foods. Cox et al. reported that dietary β -glucan down-regulated the expression of IL-8 in broiler chicks. In the present study, L. rhamnosus GG, B. breve (FRP 334), and P. pentosaceus (FRP 243 and FRP 244) combined with β -glucan from barley had the ability to reduce the IL-8 secretion when they incubated with HT-29 cells, as compared to probiotics alone. Since our previous study confirmed that β -glucan does not promote the growth of tested probiotic bacteria, however, certain probiotics were able to utilize β -glucooligomers with varying degrees of polymerization. As such, the immunomodulatory effects of the synbiotics observed in the current study with β -glucan may be due to other mechanisms that warrant further investigation. Van Zentan et al. demonstrated the limitations of pure culture screenings, whereby no growth enhancement of L. acidophilus NCFM was observed in pure culture fermentations; however, hydrolyzed oat β -glucan in simulated colonic conditions was able to support growth of NCFM. These results suggest that prebiotic effects may be more pronounced in the presence of complex microbiota found within the gastrointestinal tract. As such, future investigations with β -glucans should consider the benefit of using colonic models to confirm pure culture fermentation results. Additionally, the variability in structure among the various sources of β -glucan may contribute to variations in functionality of the product with respect immune response.
Numerous studies indicated that probiotics can modulate immunity in humans [37]. In the present study, the 7 tested probiotic bacteria showed differing abilities, even within the same species, to affect the production of IL-8 when stimulated HT-29 cells were investigated alone or combined with prebiotics. The observations are consistent with previous reports whereby the capability of these probiotic bacteria to modulate immune response was strain dependent and probiotics of the same species might have opposite effects [38]. Interestingly, in present study, B. adolescentis (ATCC 15703) consistently increased IL-8 secretion when the probiotic incubated alone with HT-29. However, the synbiotics of ATCC 15703 with raffinose, stachyose, soy extract and oligomate 55NP significantly reduced the production of IL-8 from HT-29 cells, which demonstrated that the combinations of ATCC 15703 with the above-mentioned oligosaccharides have an effect on modulating the immune system.
B. breve
Different Bifidobacterium species are capable of metabolizing complex oligosaccharides. In this study, B. breve (FRP 334) consistently demonstrated a significant decrease in IL-8 secretion when combined with all the prebiotics tested. The varying extent of oligosaccharide metabolism observed among the bifidobacteria in this study potentially reflects the number of genes encoding for metabolic enzymes for the complex oligosaccharides. For instance, β -galactosidase is a widespread enzyme found within bifidobacteria, however, Goulas et al. illustrated that bifidobacteria demonstrates diverse substrate specificities thereby affecting the types of GOS hydrolyzed by various bifidobacteria.
Significant research efforts have focused on the identification of prebiotics which can stimulate growth and/or metabolic activity of probiotics, however, the exact mechanisms involved in eliciting immunomodulatory effects remains a developing discipline which will direct future therapeutic therapies. In particular, limited research exists on the benefits of synbiotic combinations, as compared to administration of probiotics alone, and the exact mechanism(s) involved with host immune modulation. The presented data suggest that the synbiotics of GOS-type oligosaccharides with selected probiotics have a positive effect on immunomodulation by reducing the secretion of the pro-inflammatory cytokine IL-8. The majority of published research has focused on prebiotic effects of inulin, FOS and GOS, whereas our research on synbiotics of RFOs or soy extract represents novel research on the immunomodulatory effects of these synbiotic combinations. Additionally, the results of this study provide evidence that RFOs and other members of GOS have the potential roles in modulating the immune response when they combined with probiotics, though the exact mechanisms underlying requires further study. Other immunomodulatory effects of these synbiotic combinations of prebiotics with probiotics may also exist and thus, further investigations are needed.
Financial support by the Agriculture and Agri-Food Canada (AAFC) and China Scholarship Council is gratefully acknowledged.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Mei GY, Tang J, Carey CM, Tosh S, Kostrzynska M (2022) Raffinose Family Oligosaccharides and the Synbiotic with Probiotic Lactic Acid Bacteria Modulate Immune Systems: Through Inhibiting the Cytokine Production. J Prob Health. 10:288.
Received: 26-Jul-2022, Manuscript No. JPH-20-2349; Editor assigned: 28-Jul-2022, Pre QC No. JPH-20-2349 (PQ); Reviewed: 11-Aug-2022, QC No. JPH-20-2349; Revised: 18-Aug-2022, Manuscript No. JPH-20-2349 (R); Published: 29-Aug-2022 , DOI: 10.35248/2329-8901.22.10.288
Copyright: © 2022 Mei GY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.