Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research - (2023)Volume 11, Issue 4
Dietzia natronolimnaea C79793-74 have been identified as of potential probiotic strain for the management of Crohn's disease in humans. The following research presents a series of studies that establish the safety profile of this strain for use as a probiotic supplement. The genotypic characterization of Dietzia natronolimnaea C79793-74 was conducted through a combination of 16S rRNA analysis and genomic sequencing and the analysis was conducted using MiGA and MyTaxa. The safety study also involved a search of the assembled genome for the presence of antibiotic resistance genes and virulence factors using the Curated Database of Antibiotic Resistance genes (CARD) and Virulence Factor Database (VFDB) databases, respectively. Notably, Dietzia natronolimnaea C79793-74 exhibited no evidence of antibiotic resistance or virulence factors in the analysis. The utilization of D. natronolimnaea C79793- 74 as a probiotic was evaluated in an 8-week double-blind, placebo-controlled clinical trial, wherein a daily dose of 5 × 109 Colony Forming Unit (CFU) per capsule was administered to a group of healthy adult participants. This study represents the first instance in which the safety and tolerability of Dietzia natronolimnaea C79793-74 were assessed in human subjects. The results revealed that all participants, both in the dietzia and placebo groups maintained clinical and hematologic markers measured within the normal range throughout the study. Additionally, nearly all the study participants tolerated the probiotic strain well and no medium or serious adverse events were observed when compared to the placebo group. Based on the sum total of all the data obtained in this study it can be inferred that D. natronolimnaea strain C79793-74 is safe as a nutritional supplement for humans.
Dietzia; Clinical trial; Safety; Adverse effect; Probiotic
Probiotics have been defined as live microorganisms which when administered in sufficient quantities, have the capacity to enhance or restore the balance of the intestinal microbiota, resulting in various health benefits [1]. While the potential applications of probiotics in clinical settings are extensive, it is important to note that the range of clinical uses supported by evidence from rigorous clinical studies is comparatively limited [2]. Dietzia natronolimnaea C79793-74 is a nonpathogenic, non-toxin-producing bacterium that does not form spores. This microorganism belongs to the broader taxonomic group known as Dietzia sp. [3]. Strain and was originally obtained as a single colony isolate was isolated from fecal material of a paratuberculosis sero- and fecal-positive cow [4]. Johne's disease is a chronic gastrointestinal ailment caused by Mycobacterium avium subspecies paratuberculosis (MAP) in ruminants. Crohn's disease, on the other hand, is a debilitating, chronic and potentially life- threatening condition that shares significant clinical, pathological and systemic similarities with Johne's disease. It is worth noting that the role of MAP in human intestinal inflammatory diseases, such as Crohn's disease and ulcerative colitis, remains a subject of debate [5]. There is a possibility that animals afflicted with Johne's disease may serve as a source of MAP infection underlying Crohn's disease in humans [6-8]. Moreover, MAP has been implicated in an increasingly long list of granulomatous and autoimmune diseases [9].
Hence, there is a pressing need for treatments targeting MAP in the context of Crohn’s disease. Previous research has suggested that D. natronolimnaea C79793-74 could be a promising probiotic supplement to assist patients suffering from inflammatory intestinal diseases like Crohn's disease [5,10]. Furthermore, the combination of gastric acid tolerance, bile salt tolerance and resistance to lysozyme makes Dietzia natronolimnaea C79793-74 a potential candidate for probiotic use. Nevertheless, comprehensive safety evaluations are imperative. The primary objective of this study is to establish, through a safety clinical trial, that D. natronolimnaea C79793-74 can be categorized as a non-pathogenic and non-toxin- producing strain, rendering it suitable for use as a probiotic in humans.
Bacterial characterization
Bacterial strain: Dietzia natronolimnaea strain C79793-74 is a single colony isolate of a group of similar bacteria assembled under the taxon Dietzia natronolimnaea [3]. D. natronolimnaea C79793-74 was originally isolated from fecal samples on the ground of a cattle lot in River Falls, Wisconsin. The strain is deposited at the American Type Culture Collection (ATCC), USA, under strain designation PTA- 4125 with a BSL 1 in biosafety level classification [11,12].
Culture conditions of bacterial strain: A single colony was used as the inoculum for the seed culture in 10 mL of of Brain-Heat Infusion Broth (BHIB) and then incubated under aerobic conditions for 72 h. The seed culture was then diluted 1/10 into a larger volume of BHIB and incubated over a period of 3-5 days. Following this process, the bacterial cell mass is concentrated by centrifugation at 6,000 g and the resulting wet cell mass was lyophilized and milled into a fine, dry powder. Bulk powder was stored in sealed mylar bags at -20°C then encapsulated at 5 ± 0.1 billion CFU/capsule.
Genotypic characterization of the organism: Genotypic characterization of Dietzia natronolimnaea C79793-74 was carried out following 16S rRNA analysis and genomic sequencing. The C79793-74 strain was compared with ESB1503 strain [3]. L. natronolimnaea C79793-74 was sequenced and annotated by EzBiome (www.ezbiocloud.net) using published and proprietary methods. Taxonomic analysis of the strain's genomic sequence was conducted using MiGA [13].
Antimicrobial susceptibility testing: Antimicrobial Susceptibility Testing (AST) was conducted and results interpreted in accordance with the guidelines provided by the CLSI M100, 33rd. edition for the Disk Diffusion Method [14]. The following antimicrobials were used to assess the Antimicrobial Susceptibility Testing (AST) profile of Dietzia natronolimnaea strain C79793-74; ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline and chloramphenicol.
Antimicrobial resistance genes: To identify genes with high identity to previously published antibiotic resistance genes, the assembled genomes for C79793-74 were used to interrogate a CARD [15]. The database focused on acquired antibiotic resistance genes from the scientific literature and covers both gram-positive and gram-negative bacteria including pathogenic species.
The assembled genome for L. natronolimnaea C79793-74 was used to interrogate a curated database of antibiotic resistance genes CARD [15]. To support the genotypic analysis of antibiotic resistance genes, phenotypic analysis was carried out as per Clinical and Laboratory Standards Institute M02-13th Edition of Performance Standards for Antimicrobial Disk Susceptibility Tests (CLSI) Guidelines for its sensitivity/resistance against nine antibiotics; ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline and chloramphenicol [16].
Search against the virulence factor database
The assembled genome of L. natronolimnaea C79793-74 was also evaluated for the presence of known virulence factors, using the VFDB (http://www.mgc.ac.cn/VFs/) [17]. A total of 32,670 sequences were searched.
Hemolytic activity: Hemolytic Activity (HA) was measured by Hemoglobin Release (HR) as described by Lefevre et al. [18]. Briefly, human blood obtained from healthy volunteers was centrifuged (3,000 g for 5 min) to isolate the erythrocytes. The suspension of fresh erythrocytes obtained was washed and re-suspended in 1 mL of Buffered Phosphate Solution (PBS). The concentration of the suspension was adjusted by adding PBS to obtain an absorbance of 0.7 at 490 nm was obtained by adding 1 mL of the standard to 14 mL of distilled water. For the hemolysis test, different concentrations of the compound to be tested were added to the erythrocytes. After 1 h of incubation at room temperature, the samples were centrifuge and the supernatant was added in the wells of a 96-well polystyrene. The supernatant of erythrocytes in PBS1X was used as negative control and supernatant of erythrocytes in distillate water was employed as positive control. Total released hemoglobin was measured at 490 nm using an automatic absorbance microplate reader (BioTek ELx800). The percentage of hemolysis was determined using at 100% hemolysis the absorbance obtained in presence of water as follow [19].
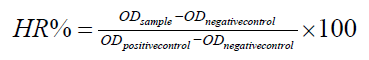
Cytotoxicity in Vero cells: Viable cells were quantified by the MTT colorimetric assay, which measures tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction by mitochondrial enzymes [20]. Briefly, 100 μL of Vero cells were seeded at 105 cells into 96-well culture plates with serum-free Dulbecco's Modified Eagle's Medium (DMEM) (Sigma- Aldrich) and treated with different concentrations of Dietzia natronolimnaea C79793–74 and Control. After 30 min incubation at 37°C and 5% CO2, 10 μl of MTT was added and the cells were incubated for three hours in similar conditions. Next, the crystals were dissolved with 100 μl Dimethylsulfoxide (DMSO) and the absorbance was measured in an ELISA reader (MRX II, Dynex Technologies) at 550 nm with the 630 nm as reference. The % of Cellular Cytotoxicity (CC) was measured as CC%=((Ac-At)/Ac) x100 where At is the absorbance of Dietzia treated cell and Ac is the absorbance of Control cells (Control are cells treated with the following excipients: calcium phosphate, magnesium stearate and colloidal silica, the excipients contained in bacteria capsule).
Human safety assessment
Study design: The present randomized, double blind, placebo controlled clinical trial study was conducted in adult, healthy volunteers. The study was designed to evaluate the safety of D.natronolimnaea C79793-74 for use as a probiotic in humans: This trial was conducted following to the good clinical practice protocols as stated in the World Medical Association Declaration of Helsinki (2013) and all procedures involving human subjects were approved under the Hermanos Ameijeiras Ethic Committee for Clinical Investigation and The Hermanos Ameijeiras Scientific Council. The D. natronolimnaea C79793-74 formulation was evaluated and approved for it use by the Ethical Committee at the National Institute of Nutrition of Cuba and The Cuba Ministry of Health.
The study enrolled 98 healthy individuals randomly assigned to either the Dietzia cohort or the Placebo group. The Dietzia cohort, consisting of 49 patients receiving the Dietzia test substance, while the Placebo group, consisted of 49 patients who were given a placebo. (Table 1) provides an overview of the demographic and other baseline characteristics of the study population. Patients were randomly assigned to treatment groups using EpiData 3.1 software. The software generated a list of random numbers for each group, which was used by pharmacy to assign individuals to their respective groups based on their compliance with the inclusion and signature criteria for informed consent. To ensure blinding, the products were dispensed in identical packaging.
| Demographic variables | Dietzia cohort (n=49) | Placebo cohort (n=49) | p value | |||
|---|---|---|---|---|---|---|
| No | % | No | % | |||
| Sex | Female | 36 | 73.5 | 31 | 63.3 | p=0.38a |
| Male | 13 | 26.5 | 18 | 36.7 | ||
| Age(years) | Median ± SD | 41.18 ± 17.14 | 44.43 ± 14.74 | p=0.29b | ||
| BMI | Median ± SD | 23.57 ± 3.69 | 23.63 ± 4.17 | P=0.94b | ||
Note: a: Fisher Test, b: t Test
Table1: Distribution of cases according to demographics and Body Mass Index (BMI).
Power and sample size consideration: The minimum required sample size for the study was estimated using a two-sided alpha of 0.05, a 95% confidence level, a standardized mean difference of 0.75 and a test power of 80%.
Subjects: The study included healthy individuals, both men and women, aged over 18 years, without distinction of sex or skin color and with hematological, clinical chemistry and hemodynamic parameters within normal ranges. Before participating in the study, all volunteers provided signed, written informed consent. Exclusion criteria included individuals suffering from any comorbidity or showing alterations in their hematological, clinical or hemodynamic parameters.
A total of 98 healthy volunteers were randomly allocated to either the Dietzia natronolimnaea (Dietzia) or Placebo groups, with 49 individuals in each group. The assignment to treatment groups was randomized using the Epidemiological Analysis from Tabulated Data (EpiData 3.1) software (Figure 1).
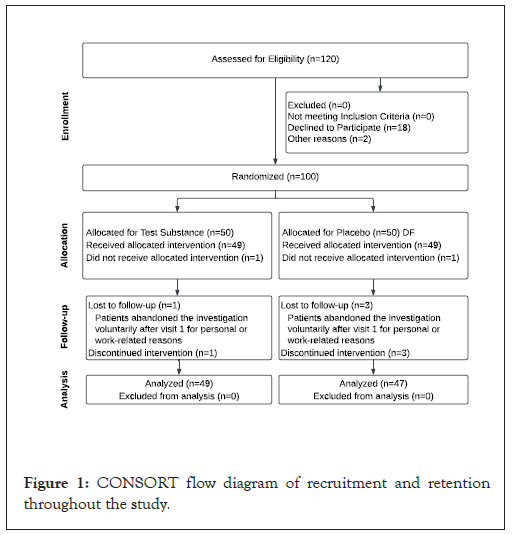
Figure 1: CONSORT flow diagram of recruitment and retention throughout the study.
Trial intervention: The study spanned a total duration of 8 weeks. All participants were instructed to orally consume one capsule daily while fasting, 40 minutes before their breakfast. They were assigned to either the D. natronolimnaea C79793-74 (Dietzia) test substance or a Placebo. Sample packages, each containing 60 capsules, were distributed over the 8-week study period. During this period, subjects in the treated group (n=50) ingested one capsule of Dietzia natronolimnaea C79793-74, which contained 5 × 109 bacteria per capsule mixed with the excipients calcium phosphate, magnesium stearate and colloidal silica. Participants in the Placebo group (n=50) were provided with capsules containing only the excipient mix.
Hemodynamic parameters, encompassing arterial pressure and heart rate, were assessed on the first day of the study, prior to probiotic consumption and at the conclusion of the study, at week 8. Additionally, a health questionnaire was administered at the baseline day preceding the commencement of the trials (week 1) and at the end of the study (week 8). (Cuestionario de Salud SF-36 (version 2) [21].
Sample collection, processing and data management: Participants underwent sample collection, supplement delivery and clinical evaluation at the beginning (week 1) and the end of the study (week 8). Samples were collected by venipuncture, then properly identified with the inclusion number, processed and aliquoted within one hour for storage and future use. All records were maintained in a dedicated database. Access to these records was limited to study and clinical staff responsible for patient care. The HHA was responsible for managing the security of the information technology infrastructure (Figure 1).
Safety assessment
Clinical determinations: Hematology parameters for obtaining standard complete blood count data were assessed through the utilization of a hematologic complex autoanalyzer XN-350 (Roche Diagnostics). This analysis was performed in strict adherence to the manufacturer's guidelines, with blood samples collected in K3 EDTA tubes at the baseline (one week before the study commencement) and at the study's conclusion (week 8).
For the evaluation of clinical chemistry parameters, a Cobas 600 modular immunochemical autoanalyzer (Roche Diagnostics) was employed. The analysis was performed on serum samples, following the manufacturer's recommended protocols.
Occurrence of adverse events determination: The occurrence of adverse events was documented by the investigators in the case report forms for each subject adhering to the framework outlined by Lefvre et al. [18]. The relationship between each adverse event and the subject's involvement in the study was assessed and categorized as improbable, possible, probable or definite. Additionally, the investigators ranked the severity of each adverse event as mild (with no impediment to daily activities), moderate (resulting in partial limitations to daily activities) or severe (rendering daily activities unattainable).
Statistical analyses: All clinical data collected were analyzed using Statistical Package for Social Sciences (SPSS) version 23.0. Descriptive statistics were used to characterize the samples. Qualitative variables were summarized in absolute numbers and percentages, while quantitative variables were summarized as mean and Standard Deviation (SD) for normally distributed data. To compare differences between groups according to qualitative variables, the chi-square test (χ²) or Fisher's exact test was used, while the Student’s t-test was used for age and Body Mass Index (BMI (quantitative variable).
The number of subjects with at least one adverse effect and the association of adverse events with study participation, were compared between the Dietzia and placebo groups using the Fisher's exact test. The Kappa Test was applied to evaluate the congruence in the responses to the health questionary (SF-36) in each group, at both time periods (before-after).
Species and strain identification
Dietzia natronolimnaea strain C79793-74 was identified using 16s rRNA gene as the phylogenetic marker. D. natronolimnaea strain C79793-74 can be distinguished from other closely related species of the genus Dietzia based on 16S rRNA sequence analysis. D. natronolimnaea strain C79793-74 showed closest homology at 97.74% with 100% coverage to D. natronolimnaea strain IL4-1.
Similarly, analysis of the strain identified as C79793-74 using whole genome sequences to interrogate the National Center for Biotechnology Information (NCBI) database and using the BLASTN algorithm [22] revealed that Dietzia natronolimnaea C79793-74 is most closely related to D. natronolimnaea as reported by Yumoto et al. [3].
The MiGA analysis revealed that strain C79793-74 is closest (97.77%) to the sequence of the strain of sp. strain ILA-1 (DSM 44820-NCBI tax ID 139021) [13]. A total of 67 contigs, representing 100% of the genome of 3,029,571 base pairs were analyzed, with very low level of contamination level (3.8%) and excellent quality (81%). The G+C content was determined to be 46.6231% with a G-C skew of 2.1581% and an A-T skew of 0.2951% Analysis of the assembled genome revealed a total of 2,897 predicted proteins with an average length of 297.3783 amino acids. The genome has a coding density of 85.3096%. The G+C observed was in agreement with that reported by Yumoto et al. for the type-strain ILA-1 (69.7 mol%) [3].
Antimicrobial resistance and virulence factors
The results, reported in (Table 2), indicate that Dietzia natronolimnaea strain C79793-74. Based on the reference strain results and cited susceptibility breakpoints the Dietzia natronolimnaea. Strain C79793-74 is fully susceptible to all the antibiotics tested, which are class representatives as follows; Ampicillin (beta-lactam class); Chloramphenicol (phenicol class); Clindamycin (lincosamide class); Erythromycin (macrolide class); Gentamicin and Kanamycin (aminoglycoside class); Streptomycin (Streptomycin class); tetracycline (tetracycline class including doxycycline and minocycline) and Vancomycin (glycopeptide class).
| Antibiotic | M100 Susceptible breakpoint for Staphylococcus aureus | Staphylococcus aureus ATCC 29213 | Dietzia natronolimnaea C79793-74 | ||
|---|---|---|---|---|---|
| Zone diameter (mm) | Interpretation | Zone diameter (mm) | Interpretation | ||
| Ampicillin | ≥ 29 | 41.8 | S | 77.8 | S |
| Chloramphenicol | ≥ 19 | 24.5 | S | 38 | S |
| Clindamycin | NA | 29 | S | 22.2 | S |
| Erythromycin | ≥ 15 | 28.4 | S | 35.7 | S |
| Gentamicin | ≥ 18 | 24.2 | S | 33.4 | S |
| Kanamycin | NA | 24 | S | 48.6 | S |
| Streptomycin | ≥ 18 | 17.1 | S | 33 | S |
| Tetracycline | ≥ 23 | 31.9 | S | 54.9 | S |
| Vancomycin | ≥ 21 | 18.7 | S | 42.4 | S |
Table 2: Results of the antimicrobial susceptibility assay for Dietzia natronolimnaea C79793-74.
Genomic analysis using the CARD database detected a 59.63% identity match to the Mycobacterium tuberculosis folC. Point mutations in the dihydrofolate synthetase folC gene, chromosomally located, [23] and has been shown to confer resistance to p-aminosalicylic acid or other aminosalicylates [24].
The analysis of virulence factor database in D. natronolimnaea C79793-74, showed that only two sequences had significant alignments. These genes were located on the chromosome and identified as follows; VFG052054 (embC) and VFG023381 (eccD3). There was no evidence of mobile elements in the flanking regions of the above-mentioned antimicrobial resistance gene.
Hemolytic activity and cytotoxicity
The Hemolytic Activity (HA) of D. natronolimnaea C79793-74 was concentration dependent as shown in (Figure 2). The maximum concentration (400 mg) showed the 100% of HA similar to that of the positive control. No appreciable HA was detected at concentration less than 128 mg/mL. The cellular cytotoxicity of D. natronolimnaea C79793-74 as measured in Vero cells showed a high % cytotoxicity at concentrations at and above 40 mg/mL (90.1%), however this effect decreases considerably at 10 mg/ml and less. (Figure 2).
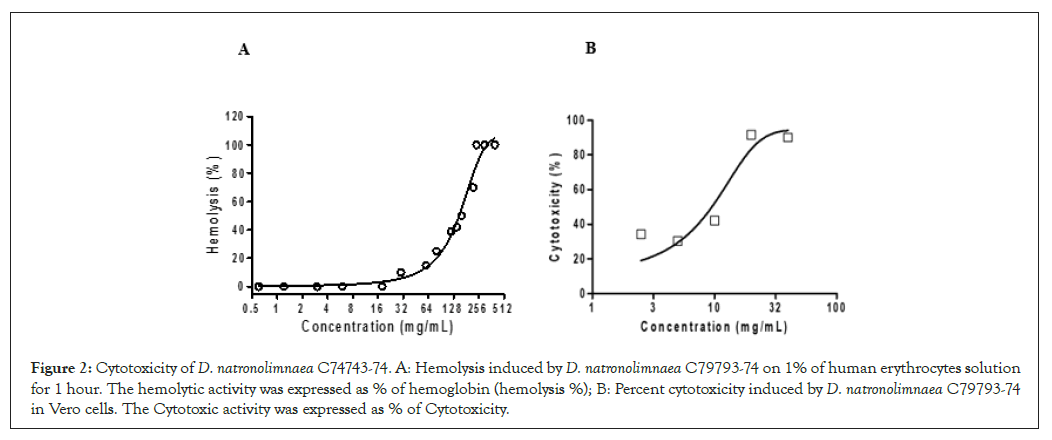
Figure 2: Cytotoxicity of D. natronolimnaea C74743-74. Note: A: Hemolysis induced by D. natronolimnaea C79793-74 on 1% of human erythrocytes solution for 1 hour. The hemolytic activity was expressed as % of hemoglobin (hemolysis %); B: Percent cytotoxicity induced by D. natronolimnaea C79793-74 in Vero cells. The Cytotoxic activity was expressed as % of Cytotoxicity.
Clinical determinations
Clinical and hematological determinations, as well as hemodynamic parameters showed normal ranges in all individuals and no differences were found between the probiotic Dietzia and Placebo cohorts (Table 3).
| Clinical chemistry and hematology | Normal range | Dietzia group | Placebo | ||
|---|---|---|---|---|---|
| (n=49) | (n=50) | ||||
| Baseline | Day 60 | Baseline | Day 60 | ||
| Creatinine | 47.6-113.4 µmol/L | 64.84 ± 15.5 | 78.63 ± 18.19 | 73.10 ± 21.59 | 76.167 ± 17.84 |
| Urea | <8.3 mmol/L | 3.34 ± 0.99 | 3.96 ± 1.46 | 4.02 ± 1.16 | 4.47 ± 1.12 |
| ALAT | <45 U/L | 17.41 ± 14.20 | 13.77 ± 4.73 | 19.44 ± 9.45 | 16.26 ± 6.71 |
| ASAT | 40 U/L | 16.29 ± 4.93 | 15.02 ± 5.70 | 18.13 ± 4.78 | 13.35 ± 5.01 |
| GGT | <50 U/L | 22.78 ± 33.07 | 19.92 ± 17.13 | 22.57± 16.62 | 24.80± 20.21 |
| Total protein | 60-80 g/L | 66.02 ± 3.81 | 69.40 ± 5.11 | 70.59 ± 6.22 | 70.77 ± 4.00 |
| Albumin | 35-52 g/L | 39.83 ± 4.46 | 43.45 ± 6.59 | 44.78 ± 3.38 | 44.65 ± 2.89 |
| Glycemia | 4.2-6.1 µmol/L | 4.85 ± 1.39 | 4.53 ± 0.49 | 4.74 ± 0.55 | 4.69 ± 0.46 |
| Cholesterol | 2.81-5.2 mmol/L | 4.89 ± 1.09 | 4.15 ± 1.23 | 4.95 ± 1.24 | 4.49 ± 1.11 |
| Triglycerides | 0.46-1.8 mmol/L | 1.10 ±0 .66 | 1.12 ± 0.69 | 1.29 ±0 .87 | 1.33 ± 0.69 |
| Total bilirubin | <17 mmol/L | 8.31 ± 5.33 | 7.14 ± 4.49 | 9.32 ± 5.33 | 7.62 ± 4.49 |
| Direct bilirubin | <5.1 mmol/L | 3.20 ± 1.61 | 3.06 ± 1.38 | 4.03 ± 4.59 | 2.95 ± 1.24 |
| WBC | (4.5-11) x 10⁹/µL | 6.50 ± 1.87 | 6.42 ± 1.75 | 6.26 ± 1.62 | 6.52 ± 1.96 |
| RBC | (F=4.2-5.4 / M=4.7-6.1) cells/ µl | 4.33 ± 0.41 | 4.43 ± 0.42 | 4.51 ± 0.40 | 4.56 ± 0.39 |
| HBG | (F=12.3-15.3/M=14.0-17.5 ) g/dL | 127.78 ± 12.49 | 130.90 ± 12.19 | 131.53 ± 15.45 | 132.51 ± 13.71 |
| HTC | (F=36-45/M=42-50) % | 0.40 ± 0.03 | 0.41 ± 0.03 | 0.40 ± 0.04 | 0.42 ± 0.03 |
| MVC | 80-96.1% | 93.60 ± 4.07 | 92.72 ± 4.35 | 90.79 ± 6.15 | 92.30 ± 5.53 |
| MCHC | 33.4-35.5 g/dl | 29.49 ± 1.37 | 29.39 ± 1.51 | 29.15± 2.36 | 29.02 ± 2.13 |
| PLT | (172-450) ×10³/mL | 234.39 ± 52.20 | 236.71 ± 53.22 | 261.88 ± 52.73 | 259.67± 62.52 |
| RDWCV | (11-14)% | 13.11 ± 0 .78 | 13.04 ± 0 .86 | 12.82 ± 1 .90 | 13.12 ± 1 .22 |
| MPV | (F: 12-16 / M: 14-17.4) g/dL | 10.16 ± 0.89 | 10.36 ± 0.83 | 10.34 ± 0.90 | 10.35 ± 0.80 |
| Neutrophil | 1.42-6.34 × 10⁹/L | 3.87 ± 1.45 | 3.60 ± 1.27 | 3.36 ± 1.16 | 3.66 ± 1.56 |
| Lymphocytes | 0.71-4.53 × 10⁹/L | 1.94 ± 0.78 | 2.07 ± 0.59 | 2.12 ± 0.63 | 2.10 ± 0.66 |
| Monocytes | 0.14-0.72 × 10⁹/L | 0.54 ± 0.18 | 0.54 ± 0.20 | 0.52 ± 0.13 | 0.52 ± 0.16 |
| Eosinophils | 0-0.54 × 10⁹/L | 0.17 ± 0.16 | 0.16 ± 0.14 | 0.20 ± 0.17 | 0.19 ± 0.13 |
| Basophils | 0-0.18 × 10⁹/L | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Hemodynamic parameters | Dietzia Group | Placebo | |||
| (n=49) | (n=50) | ||||
| Baseline | Day 60 | Baseline | Day 60 | ||
| Systolic arterial pressure (mmHg) | 119.08 ± 12.40 | 113.55 ± 20.30 | 118.39 ± 12.54 | 115.82 ± 11.41 | |
| Diastolic arterial pressure (mmHg) | 74.45 ± 7.86 | 73.27 ± 6.91 | 72.33 ± 9.08 | 71.37 ± 8.96 | |
| Heart rate (bpm) | 80.51 ± 11.19 | 80.38 ± 9.88 | 76.43 ± 12.26 | 75.51 ± 12.02 | |
Note: ALAT: Alanine Transaminase; ASAT: Aspartate Transaminase; GGT: Gammaglutamyl Transferase; WBC: White Blood Cell Count; RBC: Red Blood Cell Count; HBG: Hemoglobin, HTC: Hematocrit; MCV: Mean Corpuscular Volume; MCHC: Mean Corpuscular Hemoglobin Concentration; PLT: Platelet; MPV: Mean Platelet Volume; RDWCV: Red Cell Distribution with (Variation in the size of red blood cells); F: Female; M: Male. All values expressed as mean ± SD.
Table 3: Clinical, hematological determinations and hemodynamic parameters.
Occurrence of adverse effect determination
The study involved a total of 98 individuals, 49 in each study group. The majority of individuals, 88.8% (n=87) did not show any Adverse Effects (AE). Of these, 83.7% belonged to the Dietzia cohory (n=41) and 93.9% to the Placebo cohort (n=46). No significant differences were observed between treated group and placebo according to the development of AE (p=0.199) (Table 4). Three of the cases with AE belonged to the Placebo group. Two of them stated that they had looser stools than usual. The AE in the third case (edema) coincides with the debut of a dengue infection, so probably it could be associate to this arbovirus. Of the cases that received the Dietzia probiotic capsule, 8 individuals (16.3%) manifested some AE. The advance effect refereed were; Gastrointestinal pain or discomfort (n=5), gas (n=10), anxiety, depression (n=2), bruising (n=1), dizziness and nausea (n=4), pain headache (n=2), pain followed by capsule consumption (n=4), insomnia (n=2) (Table 5). The study involved 67 females and 31 males. Most of the advance effect were manifested by female (7/67) in relation to male (4/31). The moderate intensity was manifested in 1 female (7%) from Placebo group who debut with dengue infection and the rest of individuals do not refer any restriction of daily activities that is in correspondence with mild intensity and the effect decrease during the days of treatment (Table 4).
| Presence of AE | Dietzia cohort | Placebo cohort | Total | P value | ||
|---|---|---|---|---|---|---|
| (n=49) | (n=49) | |||||
| No | % | No | % | No (%) | ||
| No AE | 41 | 83.7 | 46 | 93.9 | 87(88.8) | 0.199 |
| AE | 8 | 16.3 | 3 | 6.1 | 11(11.2) | |
| Total | 49 | 100 | 49 | 100 | 98(100) | |
Table 4: Association of Adverse Events (AE) with study participation groups.
| Adverse event | (n=49Dietzia cohort(n=49) | Placebo cohort(n=49) | ||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Abdominal /GI discomfort | 5(4.1) | 0 | 0 | 0 | 0 | 0 |
| Acne rosacea | 0 | 0 | 0 | 0 | 0 | 0 |
| Anxiety depression | 2(2.0) | 0 | 0 | 0 | 0 | 0 |
| Joint pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Bronchitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Excision of birthmarks | 0 | 0 | 0 | 0 | 0 | 0 |
| Bruises after a fall | 1(1.0) | 0 | 0 | 0 | 0 | 0 |
| Crotid stenosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Cataract surgery | 0 | 0 | 0 | 0 | 0 | 0 |
| Chondrocalsinosis | 0 | 0 | 0 | 0 | 0 | 0 |
| Colonoscopy and fibroscopy | 0 | 0 | 0 | 0 | 0 | 0 |
| Cystitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Dental pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 2(2.0) | 0 | 0 |
| Dizziness and nausea | 2(2.0) | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 1(1.0) | 0 | |
| Gases | 10(10.2) | 0 | 0 | 0 | 0 | 0 |
| General aches | 0 | 0 | 0 | 0 | 0 | 0 |
| Genital herpes | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 2(2.0) | 0 | 0 | 0 | 0 | 0 |
| Hemorrhoids | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 0 | 0 | 0 | 0 |
| Inflamed prostate | 0 | 0 | 0 | 0 | 0 | 0 |
| Migraine | 0 | 0 | 0 | 0 | 0 | 0 |
| Mouth ulcer | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscle discomfort | 0 | 0 | 0 | 0 | 0 | 0 |
| Nasal obstruction | 0 | 0 | 0 | 0 | 0 | 0 |
| Orthopedic pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain following capsule consumption | 4(4.1) | 0 | 0 | 0 | 0 | 0 |
| Palpitations | 0 | 0 | 0 | 0 | 0 | 0 |
| Radio-infiltration (shoulder) | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhinitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Sore throat | 0 | 0 | 0 | 0 | 0 | 0 |
| Tracheitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Trouble sleeping (insomnia) | 2(2.0) | 0 | 0 | 0 | 0 | 0 |
| Vaginal dryness | 0 | 0 | 0 | 0 | 0 | 0 |
| Vagal seizures during or after taking a blood sample | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitamin D deficiency | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | 0 | 0 | 0 | 0 | 0 | 0 |
Note: GI: Gastrointestinal; n: Number of subjects. Data represent total number of adverse events followed by % in brackets.
Table 5: Summary of all advance effect by intensity.
Health questionnaire analysis
The survey questions related to the physical and emotional health status of the study subjects were analyzed (SF-36, Questions 1, 6, 7 and 11). In both groups, the behavior of the answers to these questions was very similar. Both the placebo group and the treatment group maintained similar responses at both times (Kappa ~1, p=0.000). The significant p values represent that the agreement between the responses of the two moments is high in both evaluated groups.
Dietzia natronolimnaea C79793-74 is a promising probiotic bacterium with various potential applications for Crohn's disease in humans [5,25]. However, before considering its use as a probiotic in humans, it is essential to conduct a thorough safety evaluation [26]. This safety assessment encompasses in vitro and in vivo parameters, providing a comprehensive evaluation of the strain’s safety. Morphological and cultural techniques confirmed that the strain D. natronolimnaea C79793-74 represented a pure culture exhibiting characteristics akin to those of other members within the Dietzia genus [27]. The colonies of different Dietzia strains are characterized by their small size, circular shape and convex form [28]. This is in concordance with D. natronolimnaea C79793-74 and confirm that this bacterium is a nonpathogenic, non-toxicogenic non-spore spore-forming bacterium [3].
The genotypic characterization of Dietzia natronolimnaea C79793-74 was made based on 16s RNA [28], whole genome analysis, including using the Microbial Genomes Atlas (MiGA) [13] for comparison, classification and analysis of the genome of D. natronolimnaea C79793-74, to assess its diversity, evolutionary relationships and functional potential. Both analytic methods are in concordance to strain C79793-74 is closest to the sequence of the strain of sp. strain ILA-1 (DSM 44820-NCBI tax ID 139021). This results also were confirmed by the determination of mol G+C% [13,28]. The homology-based bioinformatics framework MyTaxa [29] also showed that de strain C79793-74 falls well within the expected taxonomic affiliation for the genus Dietzia and in concordance with that of other Dietzia natronolimnaea strains.
The safety assessment for a potential probiotic strain must consider its potential to transfer antimicrobial resistance to other bacteria or to express virulence or toxin genes [26]. As an integral part of this assessment, the genome of D. natronolimnaea C79793-74 was used to interrogate the CARD [30] database for the presence of any genetic elements in its genome that showed perfect or strict matches to antimicrobial genes therein.
The analysis of the D. natronolimnaea C79793-74 genome revealed a 59.63% identity match to the Mycobacterium tuberculosis folC. Point mutations in the dihydrofolate synthetase folC gene shown clinically to confer resistance to P-Aminosalicylic acid (PAS) or other aminosalicylates. Mutations in folC inhibit bioactivation of PAS and thus confer resistance [24]. This gene is chromosomally located [23] and it is unlikely to be transferred to other bacteria. While chromosomal DNA segments can be transferred via transformation and transduction, this transfer is inefficient and random.
Phenotypic expression of antimicrobial resistance genes was also evaluated using the disk diffusion test for the presence of antimicrobial resistance traits as recommended by CLSI M100 guidelines. The results of this evaluation revealed no phenotypic resistance traits against the antimicrobials tested, which represented a broad spectrum of antimicrobial classes. These results were congruent with those obtained from the genome analysis using the CARD database. Based on these observations it is safe to say that based on the results reported herein, that D. natronolimnaea C79793-74 poses little or no risk of transmission of antimicrobial resistance and as such safe for use in food and supplements for humans [31].
The search for virulence genes using the VFDB within the D. natronolimnaea C79793-74 genome revealed the presence of two important genes; embC, which involved in the biosynthesis of mycolic acids and eccD3, a component of the ESX-3 secretion system [32,33]. These genes are of significance as they contribute to the bacterium's cell wall integrity, protection against environmental stresses and potential interactions with the host [34-36]. On the other hand, as there is no evidence of mobile elements in the flanking regions of the above-mentioned antimicrobial resistance gene, Dietzia sp. C79793-74 does not contain any sequences/genes in the genome that are risk associated, thus confirming the safety of the strain through the genome-based approach [36-38].
The cytotoxicity of probiotic strains is a distinctive attribute. Most probiotic strains either demonstrate cytotoxic effects or do not, based on their characteristic targeted mode of as a probiotic strain [38-41]. This property is particularly notable in probiotics like Bacillus subtilis and Bacillus indicus [42] and L. casei strains [43], all of which hold promise as candidates for colorectal cancer management. This targeted cytotoxic effect seen in goats and cattle after 60-day of D. natronolimnaea C797793-74 treatment for paratuberculosis could explain the therapeutic benefit observed after in [44].
The results illustrated in (Figure 2) indicate that the cytotoxicity of D. natronolimnaea C79793-74 extract on both Vero cells and erythrocytes, is dose dependent, with notable cytotoxicity at 400 mg/mL on erythrocytes and at 40 mg/mL in Vero cells. These cytotoxic concentrations exceed the dose of approximately 15 mg daily used in this study. These results support the hypothesis that at the recommended levels, D. natronolimnaea C79793-74 is safe from cytotoxic effects. This observation is supported by the lack of moderate or serious adverse events reported during this study (Table 4).
It is generally recognized that microorganisms used as nutritional supplements must be evaluated for safety in a controlled clinical trial such the one reported here. Even though Dietzia is generally regarded as nonpathogenic and a safety level 1 organism, certain species of this genus have been identified as a potential human pathogen in immunocompetent individuals and immunocompromised patients [25,45]. None of these reports identify adverse events associated with D. natronolimnaea. However, most reports recognize the useful application of Dietzia in many industries, especially the medical, chemical and food industries [5,27,46,47]. Taking in to account the potential role of Dietzia spp. as opportunistic pathogens, this clinical trial was designed to evaluate the safety of daily consumption of D. natronolimnaea. C79793-74 (5 × 109 bacteria/day-15 mg dry cell weight) for 60 days [48,49].
The demographic characteristic of the volunteers, the duration of study and the compliance to product consumption were similar for Dietzia and Placebo groups. The clinical and hematological determinations reported in (Table 2), indicated that all clinical and hematological paraments remained within the generally accepted normal range in both cohorts throughout the study. These results confirmed the healthy status of all participants and thus, supports the hypothesis that D. natronolimnaea C79793-74 is safe for human consumption at the recommended level, at least in adult’s immunocompetent individuals and could be used as a safe probiotic supplement.
Lack of understanding regarding the mechanism of action of probiotic microbes has been recognized as a hindrance to ensuring the safety of their applications [50]. However, based on the similarities of Johne´s Disease in ruminants and Crohn´s Disease in humans, the role of MAP in the pathogenesis of both of these inflammatory diseases, the inhibition of growth of MAP by Dietzia under specific culture condition and the safe used of Dietzia of cattle [5,44,46], provide supportive evidence for the safe and effective use of Dietzia in the treatment of Crohn´s and other humans Inflammatory Bowel Diseases (IBD) [5,27,47,51]. To date, there is insufficient evidence to demonstrate that the administration of probiotics existing in the market can be helpful in maintaining remission in patients with IBD [26,50]. The principally reported serious AE of probiotics are systemic infections, gastrointestinal side effects, skin complications, inflammation of endocardium, gene transfer from probiotics to the normal microbiota, metabolic harmful impacts of probiotics and immune system stimulation [50].
The analysis of AE and their intensity in this study revealed that two individuals who consumed the probiotic capsules experienced gastrointestinal discomfort, gas and pain during weeks 1 and week 3. Although they reported a decrease in the intensity of these symptoms from week 1 to week 3, they chose to discontinue the study. On the other hand, the remaining individuals in the Dietzia cohort reporting AE (Table 4) described their AE as mild and indicated that the symptoms improved within a few days. Furthermore, they reported benefits from taking the test substance, including the regulation of daily stool frequency with softer stools, improvements in low back pain and the absence of migraines. All participants reported feeling better after receiving treatment. The analyses conducted using health surveys administered before and after capsule consumption indicated that the overall health status of the treated group was not adversely affected compared to the control group. This is consistent with the few adverse effects that were observed.
This study marks the first clinical trial conducted to assess the safety and security of Dietzia species, specifically Dietzia natronolimnaea C79793-74. Our findings demonstrate that the consumption of D. natronolimnaea C79793-74 was well-tolerated and safe for nearly all the volunteer participants involved in the study. Importantly, there were no adverse events associated with the use of D. natronolimnaea C79793-74 when compared to the placebo group. Based on the sum total of all the data obtained in this study it can be inferred that D. natronolimnaea strain C79793-74 is safe as a nutritional supplement for humans.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: García G, Soto J, Barreto J, Gutiérrez A, Soto C, Pérez AB, et al (2023) Randomized Clinical Trials Demonstrate the Safety Assessment of Dietzia natronolimnaea C79793-74 for Use as a Probiotic in Humans. J Prob Health. 11:336.
Received: 03-Nov-2023, Manuscript No. JPH-23-27833; Editor assigned: 06-Nov-2023, Pre QC No. JPH-23-27833 (PQ); Reviewed: 20-Nov-2023, QC No. JPH-23-27833; Revised: 27-Nov-2023, Manuscript No. JPH-23-27833 (R); Published: 04-Dec-2023 , DOI: 10.35248/2329-8901.23.11.336
Copyright: © 2023 García G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.