Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Review Article - (2024)Volume 12, Issue 7
Objective: Coronary Heart Disease (CHD) is one of the most common cardiovascular diseases in clinic. Ginkgo biloba Ketone Ester Dripping Pill (GKEDP) is a kind of Chinese patent medicine mainly developed by Ginkgo biloba ketone ester, widely used in treating cardiovascular diseases. This study aimed to evaluate the efficacy and safety of GKEDP combined with conventional drug therapy in the treatment of Coronary Heart Disease Angina (CHDA).
Methods: Chinese and foreign literature databases were searched by computer, and all the clinical research literature of GKEDP combined with Conventional drug therapy in treating CHDA were collected. The publication time was from the establishment of the database to September 1, 2020, without language restriction, and the type of literature was limited to Randomized Controlled Trials (RCTs). According to the inclusion and exclusion criteria, two researchers independently screened the literature and evaluated the included research quality. They would decide after discussion if any divergence. Finally, the relevant original data were extracted from the incorporated literature and statistically analyzed by Revman 5.3.
Results: Ten RCT literature was finally collected with a total sample size of 1065 cases, including 533 cases in the experimental and 532 cases in the control group. Meta-analysis showed that GKEDP combined with conventional drug therapy could significantly improve the effective rate of treatment on angina pectoris [RR=1.21, 95%CI (1.15, 1.28), p<0.00001] and reduce times of angina attack [MD=-6.17, 95%CI (8.19) p<0.01], duration of angina attack [MD=-2.38, 95%CI (-3.27, -1.49), p<0.01] and chest tightness times [MD=-1.25, 95%CI (-1.92, -0.57), p<0.01] substantially.
Conclusion: GKEDP as adjuvant therapy for CHDA can significantly improve the effective rate of treatment, reduce times of angina attacks, duration of angina attacks, and chest tightness times, indicating that it can enhance the curative effect, improve symptoms and quality of life. The quality of research included in this study was low and the sample was small. Other RCTs with strict design, long treatment course, and large samples were needed to confirm these results.
Ginkgo biloba; Angina pectoris; Coronary heart disease; Coronary artery atherosclerotic heart disease; Ginkgo biloba ketone ester dripping pill
Coronary Heart Disease (CHD) is one of the most common cardiovascular diseases in clinic, with myocardial ischemia as its main feature [1]. Currently, about 110 million people suffer from CHD worldwide [2]. CHD is the leading cause of death and disability [3,4]. Angina Pectoris (AP) is the most common clinical manifestation of CHD [5], and about 90% of patients suffered from CHD will have AP [6]. AP is divided into stable AP and unstable AP. AP is primarily oppressive pain, leading to acute myocardial infarction and even sudden cardiac death [7]. It seriously affects the life safety of patients, which increases the public health burden and restricts socioeconomic development [8].
The conventional drug treatment of AP is mainly antithrombotic, reducing myocardial oxygen consumption and relieving AP [9,10], which has certain benefits, but there are still shortcomings. Approximately one-third of patients are poorly treated [11]. Long-term drug therapy is poorly tolerated and has many adverse effects, which affect the compliance of treatment and the quality of life of patients [12-14]. How to improve the curative effect and symptoms is an urgent problem to be solved.
As an adjuvant therapy, Traditional Chinese Medicine (TCM) is widely used in the treatment of AP. Coronary Heart Disease Angina (CHDA) belongs to the category of "chest paralysis" and "heart pain" in TCM. According to TCM theory, blood stasis is the main feature of CHDA, which should be treated with herbs to promote blood circulation and remove blood stasis. Ginkgo biloba, a TCM with the efficacy of activating blood circulation and removing blood stasis, has been used for centuries to treat cardiovascular diseases [15].
GKEDP mainly contains Ginkgo biloba extract [16], and clinical research shows that it has good curative efficacy and safety on CHDA. Modern pharmacological studies have shown that Ginkgo biloba extract can antagonize platelet-activating factors, lower blood lipids, and prevent the formation of atheroma plaques in coronary arteries, thus improving the symptoms of CHDA [17].
Many research had confirmed the clinical efficacy and safety of GKEDP in the adjuvant treatment of CHDA [18-27]. However, most sample sizes are small, and results may be inconsistent. No studies had been conducted to systematically analyze its clinical efficacy and safety. This study aimed to collect relevant published clinical studies for systematic analysis to assess the effectiveness and safety of GKEDP for the adjuvant treatment of CHDA and to provide evidence for clinical practice
Literature search and search strategy
Chinese and foreign literature databases, such as Chinese National Knowledge Infrastructure (CNKI), VIP Chinese Journal Service Platform, China Biology Medicine disc (CBM), Wanfang Data Knowledge Service Platform, PubMed, WOS, EMBASE, Medline, were searched by computer. All Randomized Controlled Trials (RCTs) of GKEDP combined with conventional Western medicine in treating CHDA were collected. The search timeframe was from creating the database until September 1, 2020, and there was no language restriction.
First of all, the search expression was formulated by combining subject words with free words. Then, different free words and synonyms of the target subject terms were obtained through various channels, and the retrieval is assisted by citation tracing and manual recovery. Finally, search the clinical trial registration center to collect the clinical research that meets the requirements. Try to retrieve and obtain all relevant literature as much as possible. The retrieval strategy of Pubmed was shown in Table 1, and the retrieval methods of other databases were similar to that of PubMed.
| S.no | Search strategy |
|---|---|
| 1 | Ginkgo ketone ester drippping pills (MeSH terms) |
| 2 | Ginkgo ketone ester drippping pills (Title/abstract) |
| 3 | Angina pectoris (MeSH terms) |
| 4 | Angina pectoris (Title/abstract) |
| 5 | Coronary artery disease (MeSH terms) |
| 6 | Coronary artery disease (Title/abstract) |
| 7 | Coronary artery atherosclerotic heart disease (MeSH terms) |
| 8 | Coronary artery atherosclerotic heart disease (Title/abstract) |
| 9 | 1 or 2 |
| 10 | 3 or 4 |
| 11 | 5 or 6 |
| 12 | 7 or 8 |
| 13 | 11 or 12 |
| 14 | 9 and 10 and 13 |
Table 1: Search strategy in PubMed up till September 1, 2020
Literature inclusion criteria
Any literature would be collected if they meet the following criteria. 1. The type of research was an RCT, blinded or nonblinded; 3. The subjects were of any age or sex; 2. A clinical diagnosis of CHDA was sufficient, and the diagnostic criteria were not limited; 4. In terms of interventions, the control group was treated with conventional western medicine, the observation group was treated with Ginkgo biloba drops on top of the control group, and the type and dose of western medicine were not limited; the kind of language was unlimited.
Literature exclusion criteria
Any literature will be removed if they meet the following criteria. 1. The study subjects were combined with myocardial infarction, arrhythmia etc; 2. The type of literature was non-RCTs, such as semi-randomized, controlled trials, case-control studies, cohort studies etc; 3. Literature with inaccessible full text, incorrect original data, duplicates, or unextractable; 4. Conference literature, abstract literature, animal experiment type literature, reviews, systematic analysis, case study reports, and meta-analysis; 5. No control group or the control group was non-conventional Western medicine therapy; The observation group was non- Western medicine combination therapy or didn't include GKEDP.
Outcome assessment indicators
The outcome evaluation indicators of this study included primary outcome indicators and secondary outcome indicators. The primary outcome indicator was the effective rate of treatment. The secondary outcome indicators were the times of angina attacks, the duration of angina attacks, and the times of chest tightness.
Literature selection
The literature selection for this study was done by two researchers. The two researchers independently evaluated and screened the literature according to the inclusion and exclusion criteria. After the screening process was completed, the two researchers compared the literature obtained from their respective screenings. Whether the literature with different judgment results was included or not was decided by two researchers through consultation.
Literature quality evaluation and data extraction
The extracted data is used to reflect the essential characteristics of the literature. The extracted data included the first author, publication time, sample size, average age, intervention measures, course of treatment, and outcome indicators. The methodological quality of included studies was assessed using the Review Manager (RM) 5.3 software, an RCT bias risk assessment tool provided by Cochrane Collaboration. The risk assessment included seven main aspects: The way of randomization; whether allocation concealment was used; whether the trial subjects and trial conductors were blinded; whether the study outcome assessors were blinded; the completeness of the outcome data; the selective reporting of study results; and other biases. Two investigators independently evaluated each of these seven areas and gave a judgment of "low risk of bias", "uncertain risk of bias", or "high risk of bias". In case of disagreement between the two investigators, the decision was made after their consultation.
Statistical analysis
Statistical analyses were performed using Cochrane Review Manager (RM) 5.3 software. If the analyzed indicators were categorical variables, the relative Risk Ratio (RR) and its 95% confidence interval were used, such as the treatment efficiency in this study was a categorical variable, which could be assessed by the Risk Ratio (RR) with 95% Confidence Interval (CI); if the analyzed indicators were continuous variables, the Mean Difference (MD) and its 95% confidence interval were used, such as the number of angina attacks, attack duration, and the times of chest tightness were continuous variables in this study, which could be assessed by the Mean Difference (MD) with 95% Confidence Interval (CI). Their significance was analyzed by Z test. The results of statistical analysis were considered statistically different between-group comparisons at p<0.05. The heterogeneity of studies was determined by the I2 test and Q statistical analysis. The Fixed-Effects Model (FEM) was selected if the data heterogeneity was small (p ≥ 0.1, I2 ≤ 50%); the Random- Effects Model (REM) was determined if the data heterogeneity was significant (p<0.1 or I2>50%). The stability of the combined results was explored by performing sensitivity tests through the one-by-one elimination method. Potential publication bias was assessed by constructing funnel plots (Table 1).
Studies characteristics
79 related literature was retrieved from the above Chinese databases, including 27 from CNKI, 19 from VIP, 25 from Wanfang, and eight from CBM. No related literature was obtained from the above foreign language databases, and one relevant literature was obtained by other search methods such as citation tracking and manual search. During the literature screening process, 50 duplicates were eliminated, 15 were eliminated by reading the titles and abstracts, two non-RCTs, two inappropriate interventions, and one duplicate data. Finally, a total of 10 literature [9-18], were selected. The specific process and results of literature screening are shown in Figure 1.
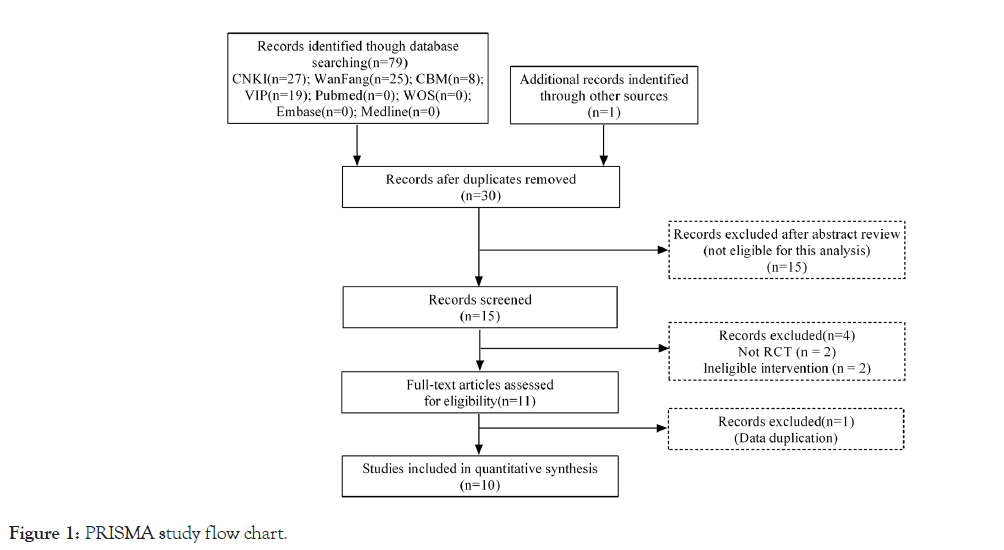
Figure 1: PRISMA study flow chart.
A total of 1065 samples were included in 10 RCTs, including 533 cases in the experimental group and 532 cases in the control group, with a maximum sample size of 136 cases and a minimum sample size of 63 cases. All the included literature described the specific course of treatment, ranging from 2 weeks to 2 months. All included literature had the effective rate of treatment as an outcome indicator, among which seven literature compare the times of AP after treatment, five literature compare the duration of AP after treatment, and two literature compare the times of chest tightness. The essential characteristics of the included studies were shown in Table 2.
| Study | Sample size(E/C) | Age/Years | Interventions | Treatment | Results indicators | |||
|---|---|---|---|---|---|---|---|---|
| E | C | E | C | |||||
| Shu-Feng Han et al. [18] | 60/60 | 60.35±3.24 | 60.28±3.17 | C+GKEDP | Routine treatment | Four weeks | ①②③ | |
| Jian-Hua Wang et al. [19] | 32/31 | 58.97±6.37 | 57.07±5.63 | C+GKEDP | Nitroglycerin | Two months | ① | |
| Zhi-Qiang Li et al. [20] | 49/49 | 58.91±4.71 | 59.71±6.52 | C+GKEDP | Routine treatment + ticagrelor | Four weeks | ①②③ | |
| Qiang Wang et al. [21] | 42/42 | 69.5±4.2 | 69.2±4.3 | C+GKEDP | Routine treatment | Four weeks | ①②③④ | |
| Quan-Yi Wang et al. [22] | 40/40 | 64±5 | 64±5 | C+GKEDP | Routine treatment + nitroglycerin | 8 weeks | ①② | |
| An-Ping Tian et al. [23] | 68/68 | 61.53±3.47 | C+GKEDP | Routine treatment + isosorbide mononitrate | 2 months | ①②③ | ||
| Mu-Sheng Feng et al. [24] | 58/58 | 62.54±7.32 | 63.58±8.13 | C+GKEDP | Routine treatment + isosorbide mononitrate | Eight weeks | ①②③ | |
| Fang Guo et al. [25] | 80/80 | 62.7±3.8 | 62.8±2.8 | C+GKEDP | Routine treatment + isosorbide mononitrate | Two weeks | ①②③ | |
| Qi Yang et al. [26] | 40/40 | 64.2±5.1 | 64.5±4.9 | C+GKEDP | Routine treatment + isosorbide mononitrate | One month | ① | |
| Jie Zhao et al. [27] | 64/64 | 62.7±4.6 | 61.5±4.4 | C+GKEDP | Routine treatment + isosorbide mononitrate | Two weeks | ①②③④ | |
Table 2: Essential characteristics of the included literature. Note: E-Experimental group; C-Control group; Results indicators: ①Effective rate of treatment; ②The times of angina attack;③The duration of the attacks; ④ The times of chest tightness
Quality assessment of included literature: All the included studies were described as adopting random grouping, among which three studies were grouped by computer numerical table method, one study was grouped by coin toss method, and the remaining six studies did not describe the random grouping method in detail. Whether the allocation scheme used concealment or the specific implementation process was not mentioned in all the included literature. All included literature had not described whether to adopt the blind method. All included literature showed complete outcome data with no reports of missed visits or dropouts. All included literature didn't describe the risk degree of other biases. The evaluation results of bias risk are shown in Figures 2 and 3.
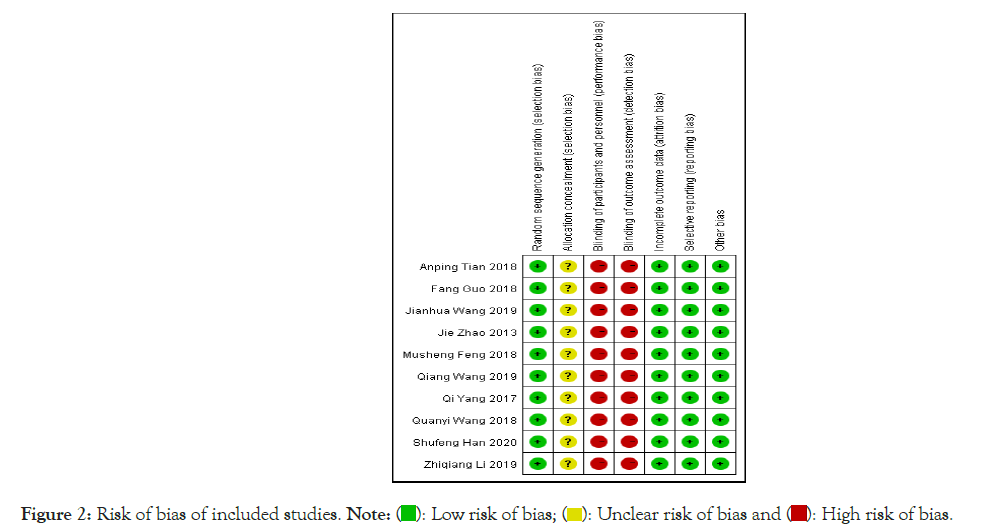
Figure 2: Risk of bias of included studies.
Note: ( ): Low risk of bias; (
): Low risk of bias; ( ): Unclear risk of bias and (
): Unclear risk of bias and ( ): High risk of bias.
): High risk of bias.
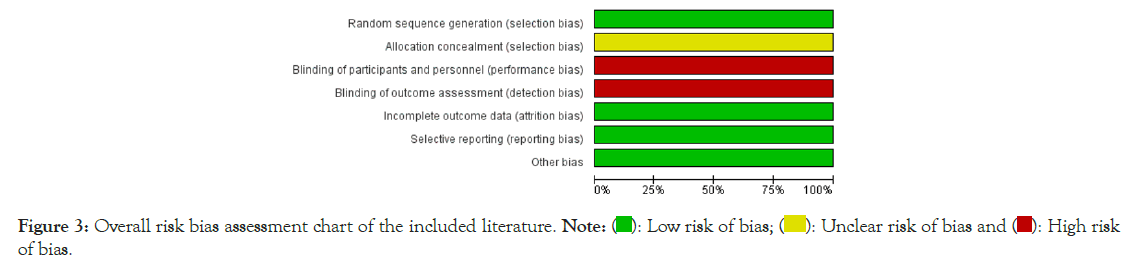
Figure 3: Overall risk bias assessment chart of the included literature.
Note: ( ): Low risk of bias; (
): Low risk of bias; ( ): Unclear risk of bias and (
): Unclear risk of bias and ( ): High risk of bias.
): High risk of bias.
The above-included studies were generally of low quality, and no attention was paid to random assignment, allocation concealment, and implementation of blinding at the time of study design. However, the performance of the blinding method had some difficulties in the above experiments. GKEDP were Chinese herbal pills. Their appearance and odor were quite different from conventional Western drugs, making it challenging to implement blinding of subjects and trial operators, which will reduce the quality of the study to some extent a certain inevitability.
Effective rate of treatment: All included studies reported the effective rate of treatment. The heterogeneity test yielded a small heterogeneity among studies (I2=0%, p=0.83>0.01). Hence, the solid-state effect model was used to combine the statistics. The results showed that the effective rate of the experimental group was higher than that of the control group after intervention [RR=1.21, 95% CI (1.15, 1.28), Z=6.76, p<0.01], as shown in Figure 4.
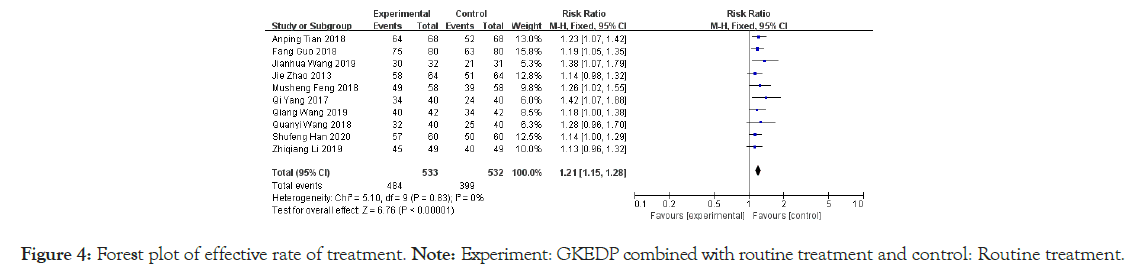
Figure 4: Forest plot of effective rate of treatment.
Note: Experiment: GKEDP combined with routine treatment and control: Routine treatment.
Times of angina attacks: The attack times of AP were reported in six studies. The heterogeneity test showed that the heterogeneity among the studies was substantial (I2=99%, p<0.01). Hence, the random effect model was used to combine the statistics. The results showed that the number of episodes of AP in the treatment group was significantly lower than that in the control group [MD=-6.17, 95% CI (-8.19, -4.14), p<0.01], as shown in Figure 5.
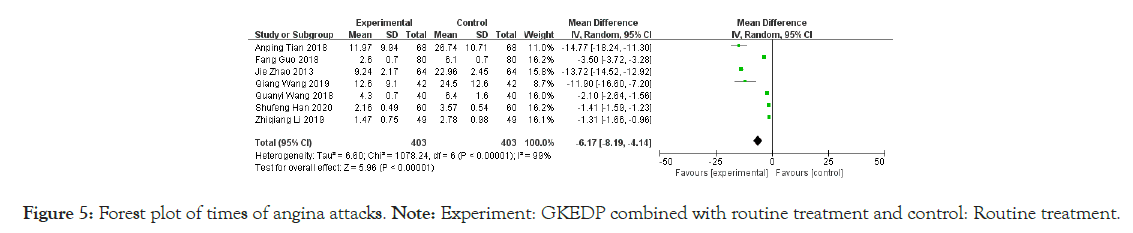
Figure 5: Forest plot of times of angina attacks.
Note: Experiment: GKEDP combined with routine treatment and control: Routine treatment.
Duration of angina attack: The duration of angina attack was reported in five studies. The heterogeneity test showed that the heterogeneity among the studies was substantial (I2=97%, p<0.01). Hence, the random effect model was used to combine the statistics. The results showed that the attack duration of AP in the treatment group after the intervention was significantly lower than that in the control group [MD=-2.38, 95% CI (-3.27,- 1.49), p<0.01], as shown in Figure 6.
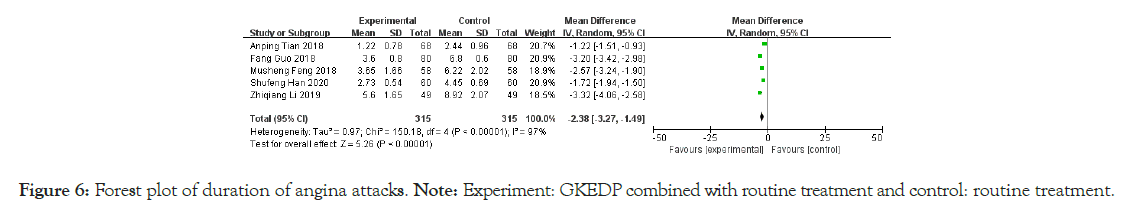
Figure 6: Forest plot of duration of angina attacks.
Note: Experiment: GKEDP combined with routine treatment and control: routine treatment.
Times of chest tightness: The times of chest tightness were reported in two studies. The heterogeneity test showed that the heterogeneity among the studies was substantial (I2=98%, p<0.01). Hence, the random effect model was used to combine the statistics. The results showed that the times of chest tightness in the experimental group after the intervention was lower than that in the control group [MD=-1.25, 95% CI (-1.92,-0.57), p<0.01], as shown in Figure 7.
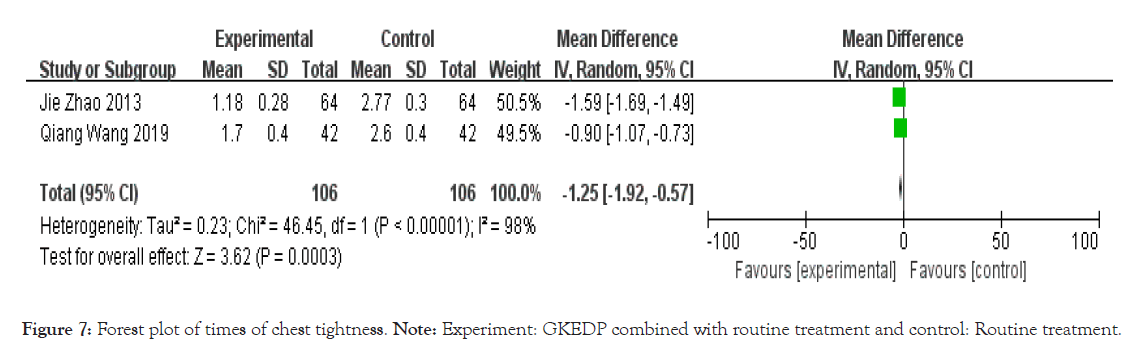
Figure 7: Forest plot of times of chest tightness. Note: Experiment: GKEDP combined with routine treatment and control: Routine treatment.
Publication bias: The publication bias in this study was examined by plotting symmetrical funnel plots, implying the absence of publication bias. In this study, funnel plots were plotted for efficiency rate of treatment, times of angina attacks, duration of angina attacks, and times of chest tightness.. The funnel plots are shown in Figures 8a-8d. Except for Figure 8b, the funnel plots were usually symmetrical, which indicated no significant publication bias.
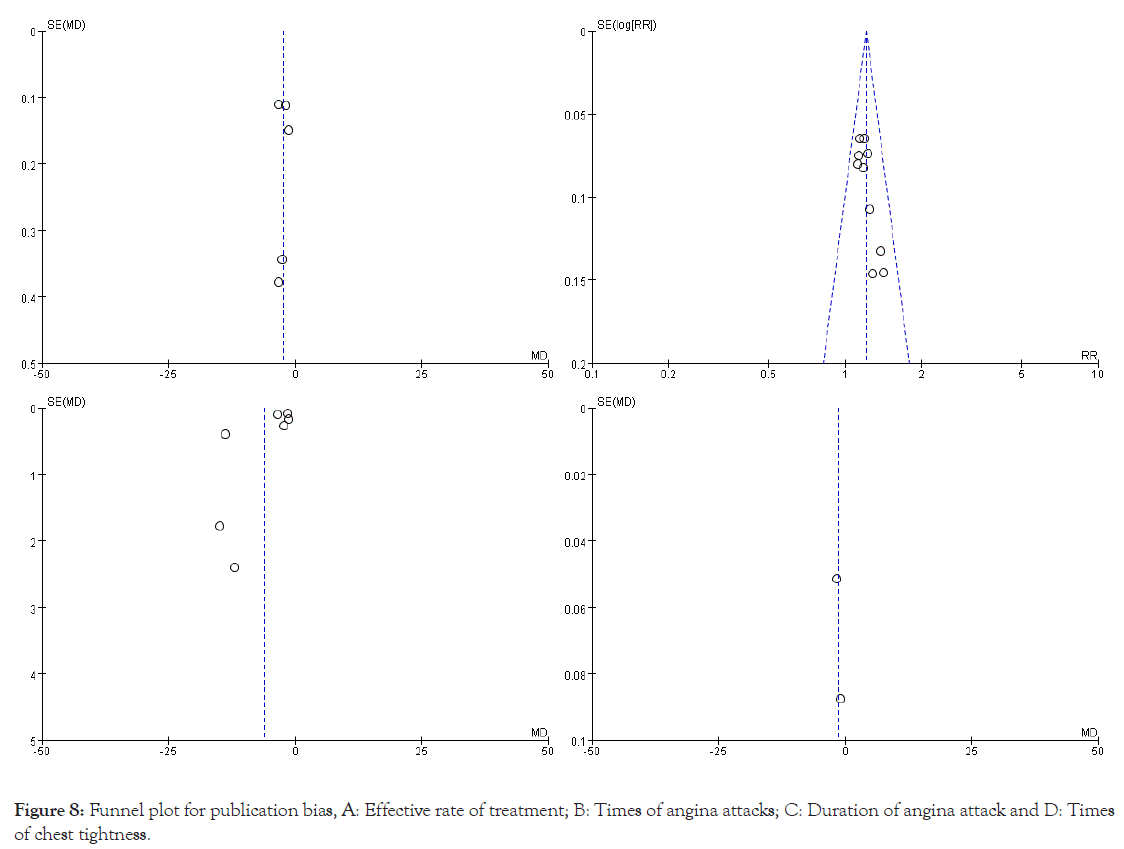
Figure 8: Funnel plot for publication bias, A: Effective rate of treatment; B: Times of angina attacks; C: Duration of angina attack and D: Times of chest tightness.
Sensitivity analysis: Sensitivity analysis was performed on all outcome indicators in the meta-analysis to explore the results stability by excluding studies one by one. Sensitivity analyses showed that the outcomes were relatively stable across studies and did not lead to a reversal of findings due to excluding specific research.
The current treatment of this disease was mainly conventional drug therapy and coronary revascularization [28]. Coronary revascularization, mainly including Percutaneous coronary Catheter Intervention (PCI) and Coronary Artery Bypass Grafting (CABG), is suitable for patients with multi-vessel and left main stem coronary artery disease [29]. PCI and CABG can significantly increase the risk of stroke, and CABG is higher than PCI [30]. PCI can improve angina symptoms substantially only within five years after operation [31]. The recurrence rate of AP was high after PCI, with approximately 20-30% of patients experiencing repeated revascularization after PCI, which dramatically increases the treatment cost [32,33]. As an invasive surgical treatment, revascularization has many contraindications and problems such as postoperative thrombosis or bleeding [34,35]. The survival rate of revascularization had no noticeable improvement for cardiovascular diseases compared with the best drug treatment, and the best drug for treatment remained the first choice [36].
GKEDP takes Ginkgo biloba ketone ester as the main component and contains flavonol glycosides, free flavonoids, terpene lactones, and other components [37]. GKEDP can inhibit platelet activation, improve endothelial function injury, and reduce liver triglyceride and liver cholesterol content, reduce plasma viscosity, and inhibit thrombosis [38,39]. Apricot ketone ester has increased arterial blood flow, inhibiting blood coagulation, reducing vascular resistance, protecting cardiomyocytes, thrombolysis, and thrombus inhibition, and can play a role in improving myocardial ischemia [40]. GDL could resist thrombosis and protect against cerebral ischemia injury through mechanisms that might involve the inhibition of platelet aggregation and the modulation of astrocytes activation through the TLR4/NF-κB signal pathway [41]. The above study proved the clinical effect of GKEDP in the treatment of CHDA from the molecular level. Ginkgo bilobalic acid is the primary toxic component of GKEDP, which has hepatotoxicity and nephrotoxicity. Its content is far lower than the relevant European standards, which may be related to the high safety of GKEDP [42,43].
This study systematically evaluated the clinical efficacy of GKEDP in the adjuvant treatment of CHDA. Meta-analysis showed that GKEDP could significantly improve the effective rate of CHDA [RR=1.21, 95%CI (1.15, 1.28), p<0.01], that is, the effective rate of CHDA in the experimental group increased by 21% compared with that in the control group. Moreover, the frequency, duration, and chest tightness of AP in the experimental group were significantly lower than those in the control group. GKEDP as adjuvant therapy can improve the effective rate, symptoms, and quality of life.
GKEDP can improve the effective rate of treatment, significantly reduce the times of angina attacks, duration of angina attacks, chest tightness, which enhanced the clinical efficacy, improved symptoms, and the quality of life. However, the literature included in this study was of low quality, with a small sample and a less rigorous design. More rigorously designed and implemented multicenter randomized controlled, double-blind trials are expected in the future to corroborate further the clinical value of GKEDP for the adjuvant treatment of CHDA.
• First, due to language barriers, we only searched Chinese and English databases.
• The overall quality of included literature was low. The implementation of randomized grouping, allocation concealment, and blinding was not described in detail and not given enough attention, which may affect the objectivity of the results.
• The total sample size of this study was small, which might lead to bias in the results.
• The diagnostic criteria adopted in some studies were not mentioned or described in detail, which might affect the reliability of the results.
• The types, doses, and frequency of medications used in some studies were not described in detail. There were many differences in age, disease duration, and treatment course of the patients in each study, contributing to more significant heterogeneity.
• Some studies had different assessment criteria for effective and ineffective in effective rate of treatment, which may affect the accuracy of the results of this study.
The data used to support the findings of this study are included within the article.
These authors declare that they have no conflicts of interest.
Xiao-Min Xue was responsible for the conceptualization of the paper, literature search, integration of the structure, and writing of the paper. Ying-Zhi Chen was responsible for the statistical analysis. Shi-Yong Chen-Jie Wang and Hui-Fang Zhou were responsible for the literature screening and quality evaluation. Si-Si Chen guided the writing of the paper and Ren-Zhao Wu reviewed and critically revised the research content for final approval for publication.
This study research was supported by grants from the Zhejiang Provincial Science and Technology Program (No. 2016F50044); the National Major Science and Technology Special Project for the Creation of Major New Drugs (No. 2016ZX09101069). The funder had no role in the study design, Literature collection, data collection, and analysis, or manuscript preparation.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Xue XM, Chen YZ, Chen SY, Li Y, Wang J, Zhou HF, et al. (2024). Randomized Controlled Trials of Ginkgo Ketone Ester Dropping Pills as Adjuvant Therapy for Coronary Heart Disease Angina: A Systematic Review and Meta-Analysis. Angiol Open Access. 12:478.
Received: 01-Jul-2024, Manuscript No. AOA-24-32544; Editor assigned: 05-Jul-2024, Pre QC No. AOA-24-32544 (PQ); Reviewed: 19-Jul-2024, QC No. AOA-24-32544; Revised: 26-Jul-2024, Manuscript No. AOA-24-32544 (R); Published: 02-Aug-2024 , DOI: 10.35841/2329-9495.24.12.478
Copyright: © 2024 Xue XM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.