Research Article - (2023)Volume 9, Issue 4
Rapid and Accurate Point of Care Diagnosis Tool to Combat an Ancient Foe
Neha Sharma1, Paras Singh1*, Monika Malik1, Sangeeta Sharma2, Khalid U. Khayyam3, Ravindra Kumar Dewan4 and Neeraj Kumar5Abstract
Tuberculosis (TB) is known to cause the highest number of infection related deaths worldwide. In 2016, WHO approved TB-LAMP (Loop-Mediated Isothermal Amplification) assay as a replacement for smear microscopy for the diagnosis of PTB in adult TB suspects. However, more epidemiological research should be conducted to support the deployment of the TB-LAMP program in peripheral level healthcare settings. This study analyzed the diagnostic efficacy of rapid and inexpensive TB-LAMP assay for the diagnosis of PTB. TB-LAMP assay displayed 1.8-fold (minimum 49.2%) higher positivity rates than the smear microscopy test (maximum 29.6%). In comparison to the composite reference standard, TB-LAMP assay was observed to be 84.3% sensitive and 96.8% specific for the diagnosis of PTB. The positive predictive value and negative predictive value of the TB-LAMP assay were 88.2 (95%CI: 77.3-94.3) and 95.6 (95% CI: 94.2-96.7), respectively. Therefore, TB-LAMP assay should be an essential point of care test for the diagnosis of PTB in adults, especially in resource limited and rural healthcare settings of TB endemic regions.
Keywords
LAMP assay; Rapid; Accurate; Molecular diagnosis; Multiplex TB PCR; Adult PTB
Introduction
Tuberculosis (TB) is a highly contagious disease with high morbidity and mortality, thus it is a major economic and health burden in developing and poor countries. In 2020, only 30 high TB burdened nations reckoned for 86% of new TB cases in which India were at top of the list [1].The World Health Organization (WHO) end TB strategy focuses to eliminate the TB epidemic worldwide till 2035, by decreasing TB prevalence and death rate to 90% and 95%, respectively [2,3]. The key to ending TB is to start early diagnosis and immediate initiation of treatment. However, in low and middle income TB endemic countries with poor healthcare settings, a large number of TB cases remain undiagnosed or misdiagnosed due to a lack of diagnostics and poor performance of available tests, which continue to fuel disease transmission and hinder TB control efforts [4]. So, globally applicable rapid diagnostic tests along with the timely intervention of treatment are crucial to ending TB. To date, TB bacteria culture remains the gold standard for TB diagnosis; however it involves long incubation time leading to a longer wait time for the test results and thereby delays the start of appropriate treatment [5]. Furthermore, sputum smear microscopy is another rapid and low cost test performed in high burden TB countries, but it has low reproducibility and only 50% to 70% sensitivity; also it cannot exclude other mycobacterial species [6]. Nucleic Acid Amplification Tests (NAATs) based methods like GeneXpert MTB/RIF and GeneXpert MTB/RIF assay, are quick, highly sensitive, and specific [7,8]. However, the high initial investment in the equipment, routinely used expensive cartridges, sophisticated laboratory facilities and need for well trained professionals restrict the use of NAAT to centralized 79 laboratories only and not in the periphery and rural areas. So, there is an urgent need to develop and deploy a highly sensitive and cheap diagnostic tool with a quick turnaround time and feasibility in resource limited regions. In 2016, WHO recommended the Loopamp™-MTBC detection test (Loop-Mediated Isothermal Amplification Assay [TB-LAMP] developed by Eiken Chemical Co. Ltd., Tokyo Japan) as an alternative to sputum smear microscopy for diagnosis of PTB in adults. The LAMP assay is based on the amplification of the Mycobacterium tuberculosis complex target genes gyrB and IS6110. TB-LAMP is another isothermal NAAT for tuberculosis diagnosis and is comparatively easier. It is more specific and sensitive than smear microscopy, less laborious, and cost-effective; quick with a turnaround time of one hour, and the final result may be visualized with the naked eye, therefore, it is appropriate for easy utility in TB epidemic resource-limited countries [9-11]. Considering its easy to use characteristics, rapid result delivery, high diagnostic efficiency, and cost effectiveness, TB-LAMP can be a major milestone in the field of TB diagnosis and will help to break off the cascade of tuberculosis infection in TB-endemic countries like India. Although many studies have been conducted in various epidemiological regions, they showed the variation of sensitivities of TB-LAMP to detect bacteria in sputum samples. So, further clinical evaluation of the LAMP in the TB epidemic Indian population is needed before implementing this test at the peripheral level. The objective of this study was to evaluate the clinical performances/diagnostic accuracy of the TB-LAMP for the diagnosis of pulmonary tuberculosis in suspected cases from Delhi and its surrounding states.
Materials and Methods
Study protocol and sample procurement
The patients suspected of having pulmonary tuberculosis and visiting the out-patient department of NITRD between October 2021 and May 2022 were enrolled in this study. This study was performed at the National Institute of Tuberculosis and Respiratory Diseases (NITRD), New Delhi, India after obtaining ethical clearance from the institutional ethics committee. All participants provided written informed consent at enrollment in this study.
A total of 466 adults with suspected PTB were enrolled in the present study.
Inclusion criteria: Adult cases (≥ 18 years of age) of any gender with clinically suspected PTB were included under the study.
Exclusion criteria: People with age <18 years and adults without the clinical symptoms of PTB were excluded from the study.
Sample processing and microbiological diagnosis of PTB
The sputum sample was collected from the suspected PTB cases attending the OPD of NITRD, New Delhi. All samples were quickly transported to the department of molecular medicine's laboratory at the NITRD for additional processing as per the various diagnostic test protocols.
NALC-NaOH decontamination: The sputum samples were decontaminated by treatment with NALC-NaOH, as per the protocol published earlier.
DNA purification
The DNA from the decontaminated sputum specimen was extracted using the Qiagen's QIAamp DNA mini kit (QIAGEN, Hilden, Germany) as per manufacturer's instructions.
Molecular diagnosis
Multiplex TB PCR for IS6110 and MPB64 genes: The purified bacterial DNA was administered to multiplex polymerase chain reaction assays for the detection of two Mtb genes, viz., IS6110 and MPB64. The reaction mixture was composed of 50 ng–100 ng of sample DNA, 2.5 mM of PCR buffer, 200 μM–400 μM dNTP mix, 50 ng–100 ng of each primer (forward and reverse), 1 U of Taq polymerase in a 25 μL of the final volume. The sequences of primers for both the genes and steps of the PCR assay are given in Table 1.
| Gene | Primer sequence (5′ to 3′) | PCR product size (bp) | PCR conditions |
|---|---|---|---|
| IS6110 | F: CTGCGAGCGTAGGCGTCGG; R: CTCGTCCAGCGCCGCTTCGG | 123 | 1 cycle of 94°C for 5 min.; 40 cycles of 94°C for 1 min., 65°C for 1 min., 72°C for 1 min.; 1 cycle of 72°C for 10 min. |
| MPB64 | F: TCCGCTGCCAGTCGTCTTCC; R:GTCCTCGCGAGTCTAGGCCA | 240 | 1 cycle of 94°C for 5 min.; 40 cycles of 94°C for 1 min., 65°C for 1 min. 30 sec., 72°C for 1 min. 30 sec.; 1 cycle of 72°C for 10 min. |
Table 1: Primer sequences and thermal conditions for the amplification of Mtb genes using conventional PCR.
GeneXpert MTB/RIF assay: The GeneXpert MTB/RIF assay (Cepheid, USA) was carried out as per the manufacturer’s instructions.
TB-LAMP assay: The TB-LAMP assay was performed using Loopamp™ MTBC detection kit. Sixty microlitres of purulent sputum samples were dispensed into the heating tubes and loaded onto the heating block at 90°C for 5 min. The tubes were cooled down for 2 minutes and screw capped to adsorbent tubes followed by vigorously shaking. The adsorbent tubes were connected to the injection caps. The caps of the injections were removed and adsorbent tubes were squeezed to dispense about 30 μL of DNA in the reaction tubes. The reaction tubes were closed and left for 2 minutes to reconstitute the dried reagents. The reaction tubes were then incubated for amplification reaction at 67°C for 40 min. The reaction was terminated by incubating the reaction tubes at 80°C for 5 min. The final results were determined by examining the reaction tubes for fluorescence under UV light or turbidity visualized by naked eyes. Positive and negative controls (provided with the kit) were incorporated in every run of the assay. The volunteers who never had symptoms or history of mycobacterial infection were contemplated as healthy controls for this assay [12].
Statistical evaluation
The composite reference standard (included smear microscopy, multiplex TB PCR and TB-LAMP assay) was taken as a reference standard for evaluating various parameters comprised of sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and accuracy using Medcalc online software. The TB positivity rate was compared by direct counting method.
Results
Study participants and clinical samples
A total of 466 patients met the inclusion criteria of our study. Amongst these sputum samples, 20 samples were not found to be appropriate for testing, and 7 samples were excluded as these depicted ‘invalid’ results on GeneXpert. In this study, out of the 439 samples analyzed, 271 (61.7%) were males and 168 (37.2%) were females. All of them were in the age range of 18 to 86 years (Figure 1). Thirty samples of healthy controls were included in the TB-LAMP assay and Multiplex TB PCR assays as negative controls.
Comparative diagnostic performance of TB-LAMP and the conventional methods
Of the total 439 clinically suspected PTB cases, only 29.6% were diagnosed as TB-positive by smear microscopy. On the contrary, 55.8% of specimens were confirmed TB-positive by molecular diagnostic-multiplex TB PCR test (Figure 2). All the samples were classified as per the ‘any positive’ rule, i.e., a samples would be considered TB positive if tested positive by any of 3 tests, whereas samples were categorized TB-negative if tested negative by all 3 tests. Upon comparison with the Composite Reference Standard (CRS),the sensitivity of TB-LAMP, multiplex TB PCR, and smear microscopy was observed to be 84.3% (95% CI: 79.2%-88.6%), 98.3% (95% CI: 95.9%-99.5%) and 49.0% (95% CI: 42.6%-55.3%), respectively. The specificity, PPV, and NPV of the TB-LAMP assay were found to be 96.8% (95% CI: 93.2%-98.8%), 97.4% (95% CI: 94.5%-98.8%), and 81.1% (95% CI: 76.2%-85.1%), respectively. The specificity, PPV, and NPV of multiplex TB PCR were observed to be 98.4% (95% CI: 95.4%-99.6%), 98.9% (95% CI: 96.6%-99.6%), and 97.7% (95% CI: 94.1%-99.1%), respectively, which are higher than those of TB-LAMP but the difference were not statistically significant. The specificity, PPV and NPV of the smear microscopy was observed to be 95.7% (95% CI: 91.8%-98.1%), 94.3% (95% CI: 89.3%-97.0%) and 56.6% (95% CI: 53.5%-59.6%), respectively, which are lesser than the TB-LAMP (Table 2). This indicated that the molecular methods demonstrated a highest positivity rate of PTB diagnosis than microbiological test.
| Diagnostic test | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI) | Negative predictive value % (95% CI) |
|---|---|---|---|---|
| TB-LAMP | 84.3 (79.2- 88.6) | 96.8 (93.2- 98.8) | 97.4 (94.5-98.8) | 81.1 (76.2-85.1) |
| Smear | 49 (42.6-55.3) | 95.7 (91.8- 98.1) | 94.3 (89.3-97.0) | 56.6 (53.5-59.6) |
| Multiplex TB PCR | 98.3 (95.9-99.5) | 98.4 (95.4- 99.6) | 98.9 (96.6-99.6) | 97.7 (94.1-99.1) |
Table 2: Comparison of diagnostic efficiency of different TB assays with CRS in PTB cases.
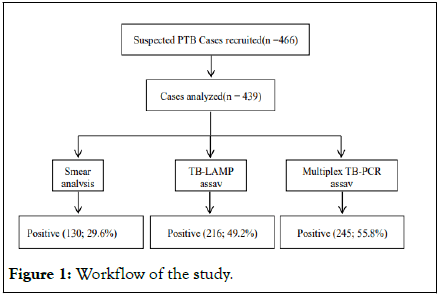
Figure 1: Workflow of the study.
Diagnostic accuracy of TB-LAMP in smear negative cases
Of the 439 specimens, 70.3% (n=309) samples were classified as smear negative. Out of these 309 specimens, 86 (27.8%) were found to be TB positive by LAMP assays and 115 (37.2%) came out to be positive by multiplex TB PCR. In our study we observed that the sensitivity and specificity of TB-LAMP in smear positive cases were 98.4% (95% CI: 94.5%-99.8%) and 99.6% (95% CI: 98.2%-99.9%); and in smear-negative cases were 57.4% (95% CI: 50.5%-64.0%) and 85.2% (95% CI: 79.8%-89.5%), respectively. Moreover, all controls were well diagnosed, so the TB-LAMP test can be contemplated as an accurate assay for identifying “hidden” PTB cases. The PPV and NPV of TB-LAMP assay in smear-positive cases were 99.7%(95% CI: 98.4%-99.9%) and 97.8% (95% CI: 91.9%-99.4%); and in smear-negative cases were 84.8% (95% CI: 79.9%-88.6%) and 58.1% (95% CI: 54.1%-62.0%), respectively (Table 3). The 30 healthy control samples were accurately assessed negative by both TB-LAMP assay and Multiplex TB PCR. So in smear negative cases, TB-LAMP seems to be an ideal PTB detection test for hidden TB cases.
| Patient group | Diagnostic test (n=439) | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI ) | Negative predictive value % (95% CI) |
|---|---|---|---|---|---|
| Smear +ve | LAMP assay vs. Smear | 98.4 (94.5- 99.8) | 99.6 (98.2-99.9) | 99.7 (98.4- 99.9) | 97.8 (91.9-99.4) |
| Smear -ve | LAMP assay vs. Smear | 57.4 (50.5- 64.0) | 85.2 (79.8- 89.5) | 84.8 (79.9-88.6) | 58.1 (54.1- 62.0) |
Table 3: Diagnostic efficiency of LAMP assay vs. smear microscopy in PTB cases.
Diagnostic accuracy of TB-LAMP and GeneXpert MTB/RIF
We were able to generate GeneXpert MTB/RIF data for only 209 samples out of 439 samples included in this study. Therefore, we compared the PTB diagnosis based on the LAMP assay with that of GeneXpert MTB/RIF in these 209 adult sputum specimens (Figure 2). The sensitivity and specificity of TB-LAMP vs. GeneXpert MTB/RIF were 92.9% (95% CI: 86.6%-96.9%) and 95.7% (95% CI: 89.5%-98.8%). The PPV and NPV values of TB-LAMP vs. GeneXpert MTB/RIF were 96.9% (95% CI: 92.4%-98.8%) and 90.4% (95% CI: 82.9-94.8), respectively (Table 4). Of the 209 cases, only 114 (54.5%) samples were diagnosed TB-positive by GeneXpert MTB/RIF and 110 (52.6%) by LAMP. Of the 114 cases, confirmed positive by GeneXpert MTB/RIF, 57 were found to be Rifampicin (RIF) sensitive, 22 were rifampicin-resistant; and, and 2 samples depicted indeterminate drug susceptibility. Out of these RIFsensitive samples, 52 cases were diagnosed TB positive by LAMP test. Also, 4 RIF resistant cases were positive by LAMP assay. The Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curves for TB-LAMP and GeneXpert MTB/RIF were 0.965 and 0.987, respectively (Figure 3).
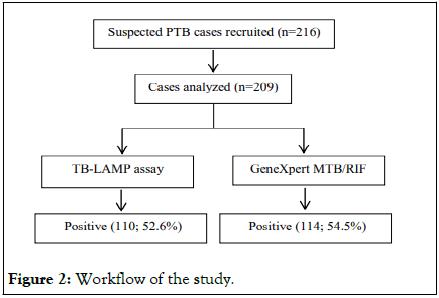
Figure 2: Workflow of the study.
| Diagnostic test (n=209) | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI ) | Negative predictive value % (95% CI) |
|---|---|---|---|---|
| TB-LAMP vs. GeneXpert MTB/RIF | 92.9 (86.6-96.9) | 95.7 (89.5-98.8) | 96.9 (92.4-98.8) | 90.4 (82.9-94.8) |
Table 4: Comparison of diagnostic efficiency of LAMP assay vs. GeneXpert MTB/RIF in PTB cases.
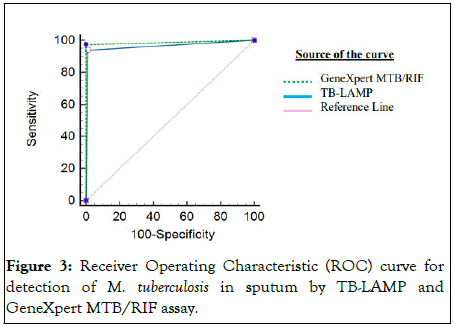
Figure 3: Receiver Operating Characteristic (ROC) curve for detection of M. tuberculosis in sputum by TB-LAMP and GeneXpert MTB/RIF assay.
Discussion
TB-LAMP was recommended by WHO in 2016 for diagnosis of adult pulmonary tuberculosis. However, it`s not yet a fundamental component of the PTB diagnosis in TB-endemic countries like India. So far very limited studies have been conducted to evaluate the diagnostic efficacy of TB-LAMP tests in various tuberculosis prevalent areas. In this study, we have evaluated the PTB diagnostic efficiency of the TB-LAMP in the TB-endemic Delhi NCR population of India.
In this study, the PTB diagnosis positivity rate of the LAMP assay was 49.6% (216 patients out of 439), being 1.8-fold higher than that of smear microscopy. Since there is a lack of any gold standard and ideal comparison for molecular assay TB-LAMP, we evaluated the diagnostic competence of the TB-LAMP test with respect to a Composite Reference Standard (CRS) which incorporated smear, multiplex TB PCR (IS6110-PCR and MPB64-PCR) and TB-LAMP [13,14]. A 1.9-fold difference was accessed in PTB diagnostic positivity rate between Multiplex TB PCR and smear microscopy in suspected cases. The specificity of all three methods was almost similar upon comparison with CRS in our study. In spite of the fact that multiplex PCR has the highest sensitivity and specificity for PTB diagnosis, it is time consuming and requires well trained technical manpower for its ideal performance. Upon comparison with CRS, we found that multiplex TB PCR demonstrated the highest sensitivity 98.4%, followed by LAMP TB assay depicting a sensitivity of 84.3%, and smear microscopy demonstrated the lowest sensitivity of 49%. However, few studies conducted in China, Vietnam, and India demonstrated contradictory results, reporting a sensitivity of TB-LAMP as low as 70.5%-79.5% [15,16]. But the majority of previous literature is in concurrence with our study that illustrates TB-LAMP assay as a rapid, cheap and efficacious diagnostic test for pulmonary tuberculosis with a sensitivity ranging between 82.9%-100% [17-22]. Among the smear positive cases, the LAMP assay demonstrated 98.4% sensitivity for PTB diagnosis. This is in accordance with earlier studies, which demonstrated the sensitivity of TB-LAMP to be 100% in smear-positive sputum samples. All these results depicted that TB-LAMP is the best supportive test for clinical diagnosis in smear-positive cases.
Further, TB-LAMP is of great significance, especially in the cases that turn out to be TB negative by smear microscopy and misdiagnosed or left undiagnosed and untreated, thereby serving as “hidden infectious TB catalysts” in spreading tuberculosis in the population. Smear microscopy is a laborious, ineffective procedure that is more prone to human error while scanning the slides. However, due to a lack of resources, smear microscopy is still used in underdeveloped nations with high TB prevalence for quick MTB detection. We observed that the sensitivity of TB-LAMP in smear-positive cases (98.4%) was very high and was almost half of it in smear-negative cases (57.4%), which was in concordance with the observation of previous studies (46.6%-58.8%). Therefore, to find the “hidden” TB cases in areas with a high TB burden and limited resources, our study will help to fulfill an urgent need to establish a simpler, more affordable, safe, quick, and efficient diagnostic procedure like TB-LAMP. So, contemplating the diagnostic efficiency and convenient operational features, TB-LAMP can be a prospective assay to fill the lagging gaps in the diagnosis of PTB, especially in countries with a high tuberculosis burden of rural and primary health centers in developing and TB endemic countries like India.
Our research and many previous studies suggest that the TB-LAMP assay is equivalent in specificity to GeneXpert MTB/RIF. Our study's sensitivity of GeneXpert MTB/RIF was 92.9% higher than the TB-LAMP assay. This may be due to the larger sample volume used in GeneXpert MTB/RIF compared to TB-Lamp [23]. The sensitivity and specificity of the TB-LAMP assay (96.9% and 96.5%) were comparable to those of the GeneXpert MTB/RIF (95.4% and 93.9%) when compared to smear microscopy [24]. A major advantage of GeneXpert MTB/RIF for TB-LAMP is its ability to detect rifampicin resistance along with tuberculosis infection. However, the GeneXpert MTB/RIF is expensive and requires sophisticated equipment and infrastructure, making them unsuitable for peripheral laboratories [25]. Despite its similarity in sensitivity and specificity to GeneXpert, LAMP molecular assay has a shorter turn-around time, minimal infrastructure, least technical training, and visual readout, So TB-LAMP can serve as a quick economical and highly sensitive point of care diagnostic tool to inhibit TB transmission, enhance treatment effectiveness, and improve the quality of life of people living in TB endemic areas, immigrants, high-risk settings, and close contact settings.
Conclusion
As, TB-LAMP satisfies the WHO requirements for an alternative test, our study presents further evidence that it surpass smear microscopy in terms of sensitivity in the diagnosis of PTB in adults. Hence, TB-LAMP might be considered as a replacement for smear microscopy. Further, our study demonstrated that the sensitivity and specificity of the TB-LAMP assay were slightly lower than that of the GeneXpert MTB/RIF.TB-LAMP assay may serve as rapid, cost effective test with the need of minimal operational infrastructure and manpower technical training for diagnosing adult PTB. Therefore, TB-LAMP may possibly a practical substitute for smear microscopy and GeneXpert MTB/RIF in environments with limited resources and inadequate infrastructure within high TB burden developing countries.
Funding
Dr. Ravindra Kumar Dewan, Director, National Institute of Tuberculosis and Respiratory Diseases, New Delhi, India provided the support for funds and materials for this work.
Competing Interests
The authors declare that they have no competing interests in the publication of this manuscript.
Acknowledgments
The authors acknowledge the patients and healthy volunteers for their contribution in providing test samples (with their consent) for this study.
References
- World Health Organization. Global tuberculosis report. WHO. 2021.
- Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393(10181):1642-1656.
- World Health Organization. The END TB strategy. WHO. 2015.
- Gill CM, Dolan L, Piggott LM, McLaughlin AM. New developments in tuberculosis diagnosis and treatment. Breathe. 2022;18(1): 210149.
[Crossref] [Google Scholar] [PubMed]
- Pai M. Tuberculosis. Nat Rev Dis Primers. 2016;27:16076.
- Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review. Lancet Infect Dis. 2006;6(9):570-581.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Tuberculosis Using the GeneXpert MTB/RIF assay to detect pulmonary and extrapulmonary tuberculosis and rifampicin resistance in adults and children. Expert group meeting report. 2013.
- World Health Organization, Tuberculosis. Next-generation Xpert®MTB/RIF Ultra assay recommended by WHO. WHO. 2017.
- World Health Organization, Tuberculosis. The use of loop-mediated isothermal amplification (TBLAMP) for the diagnosis of pulmonary tuberculosis. WHO. 2016.
- Shete PB, et al. Diagnostic accuracy of TB-LAMP for pulmonary tuberculosis: A systematic review and meta-analysis. BMC Infect Dis 2019;19(1):268.
- Rajput R, Singh P, Sarin R, Sethi P, Sharma S. Diagnostic accuracy of loop mediated isothermal amplification assay for extra pulmonary tuberculosis in Indian population. J Microbiol Methods. 2019;158:59-65.
[Crossref] [Google Scholar] [PubMed]
- Yadav R, et al. Diagnostic accuracy of TB-LAMP assay in patients with pulmonary tuberculosis-a case control study in Northern India. Pulmonol. 2022;8(6):449-453.
[Crossref] [Google Scholar] [PubMed]
- Lekhak SP, Sharma L, Rajbhandari R, Rajbhandari P, Shrestha R, Pant B. Evaluation of multiplex PCR using MPB64 and IS6110 primers for rapid diagnosis of tuberculous meningitis. Tuberculosis. 2016;100:1-4.
[Crossref] [Google Scholar] [PubMed]
- Wei Z, Zhang X, Wei C, Yao L, Li Y, Zhang X, et al. Diagnostic accuracy of in house real time PCR assay for Mycobacterium tuberculosis: A systematic review and meta-analysis. BMC Infect Dis. 2019;19:701.
[Crossref] [Google Scholar] [PubMed]
- Ou X, Li Q, Xia H, Pang Y, Wang S, Zhao B, et al. Diagnostic accuracy of the PURE-LAMP test for pulmonary tuberculosis at the county-level laboratory in China. PLoS One. 2014;9(5):e94544.
[Crossref] [Google Scholar] [PubMed]
- Pham TH, Peter J, Mello FC, Parraga T, Lan NT, Nabeta P, et al. Performance of the TB-LAMP diagnostic assay in reference laboratories: Results from a multicentre study. Int J Infect Dis. 2018;68:44-49.
[Crossref] [Google Scholar] [PubMed]
- Bentaleb EM, Abid M, El Messaoudi MD, Lakssir B, Ressami EM, Amzazi S, et al. Development and evaluation of an in-house single step loop-mediated isothermal amplification (SS-LAMP) assay for the detection of Mycobacterium tuberculosis complex in sputum samples from Moroccan patients. BMC Infect Dis. 2016;16(1):517.
[Crossref] [Google Scholar] [PubMed]
- Bojang AL, Mendy FS, Tientcheu LD, Otu J, Antonio M, Kampmann B, et al. Comparison of TB-LAMP, GeneXpert MTB/RIF and culture for diagnosis of pulmonary tuberculosis in The Gambia. J Infect. 2016;72(3):332-337.
[Crossref] [Google Scholar] [PubMed]
- Yadav R, Sharma N, Khaneja R, Agarwal P, Kanga A, Behera D, et al. Evaluation of the TB-LAMP assay for the rapid diagnosis of pulmonary tuberculosis in Northern India. Int J Tuberc Lung Dis. 2017;21(10):1150-1153.
[Crossref] [Google Scholar] [PubMed]
- Sharma G, Tewari R, Dhatwalia SK, Yadav R, Behera D, Sethi S. A loop mediated isothermal amplification assay for the diagnosis of pulmonary tuberculosis. Lett Appl Microbiol. 2019;68(3):219-225.
[Crossref] [Google Scholar] [PubMed]
- Linhas R, Oliveira O, Meireles P, Oliveira P, de Melo MB, Lourenco J, et al. Immigrants’ access to health care: Problems identified in a high risk tuberculosis population. Pulmonol. 2019;25(1):32-39.
[Crossref] [Google Scholar] [PubMed]
- Kim CK, Cho EA, Shin DM, Choi SW, Shin SY. Comparative evaluation of the loop mediated isothermal amplification assay for detecting pulmonary tuberculosis. Ann Lab Med. 2018;38(2):119-124.
[Crossref] [Google Scholar] [PubMed]
- Sethi SK, Singh S, Dhatwalia SK, Yadav R, Mewara A, Singh M, et al. Evaluation of in house Loop-Mediated Isothermal Amplification (LAMP) assay for rapid diagnosis of M. tuberculosis in pulmonary specimens. J Clin Lab Anal. 2013;27(4):272-276.
[Crossref] [Google Scholar] [PubMed]
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005-1015.
[Crossref] [Google Scholar] [PubMed]
- Creswell J, Codlin AJ, Andre E, Micek MA, Bedru A, Carter EJ, et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014;14(1):1-2.
[Crossref] [Google Scholar] [PubMed]
Author Info
Neha Sharma1, Paras Singh1*, Monika Malik1, Sangeeta Sharma2, Khalid U. Khayyam3, Ravindra Kumar Dewan4 and Neeraj Kumar52Depatrment of Pediatrics, National Institute of Tuberculosis and Respiratory Diseases, New Delhi, India
3Department of Epidemiology and Public Health, National Institute of Tuberculosis and Respiratory Diseases, New Delhi, India
4Department of Thoracic Surgery and Surgical Anatomy, National Institute of Tuberculosis and Respiratory Diseases, New Delhi, India
5Department of Pathology, National Institute of Pathology, New Delhi, India
Citation: Sharma N, Singh P, Malik M, Sharma S, Khayyam KU, Dewan RK, et al. (2023) Rapid and Accurate Point of Care Diagnosis Tool to Combat an Ancient Foe. Appli Microbiol Open Access. 9:268.
Received: 26-Apr-2023, Manuscript No. AMOA-23-23759; Editor assigned: 28-Apr-2023, Pre QC No. AMOA-23-23759 (PQ); Reviewed: 12-May-2023, QC No. AMOA-23-23759; Revised: 22-Jun-2023, Manuscript No. AMOA-23-23759 (PQ); Published: 29-Jun-2023 , DOI: 10.35248/2471-9315.23.9.268
Copyright: © 2023 Sharma N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.