Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2013) Volume 1, Issue 3
Pure culture of Pediococcus spp GS4 isolated from khadi was used as inoculum for curd preparation. 1% of the bacterial culture having viable cell count of 1.24×109 CFU/ml was inoculated, and curdling was observed after 18 hours of incubation at 37°C. The cell viability in the curdled sample was determined to be 2.46×109 CFU/ml. Physico-chemical analysis of the curd showed its moisture content to be 90.36%, free amino acids amounted to a concentration of 710 μg/μl, and protein and carbohydrate concentration in the curd was determined to be 460 μg/μl and 0.86 mg/ml, respectively. The free fatty acid content was estimated to be 6.77 g/100g as oleic acid equivalence. The confirmation of probiotic properties showed acid and bile tolerance with the percentage survivability of 88.01 and 113.33%, respectively. Antimicrobial activity of the 100 μl of the cell free extract gave maximum inhibition against Staphylococcus aureus, with the Zone of inhibition (ZOI) of 13.9 ± 0.32 mm, followed by Pseudomonas aeruginosa (12.2 ± 0.45 mm), and the least with Escherichia coli and Listeria monocytogenes with the average ZOI of 11.9 ± 0.25 and 10.6 ± 0.85 mm, respectively. The concentration of lactic acid was determined to be 2.43 ± 0.01 g/20ml of supernatant. The viable counts upon lyophilisation showed a decrease in viability and the counts dropped to less than 108 CFU/ml after the 6th day of storage at room temperature. Organoleptic evaluation of the reconstituted curd was judged as acceptable. The curd thus prepared possessed the health beneficial and organoleptic property to support and supplement the rural health and economy.
Keywords: Pediococcus spp GS4, Probiotic; Curd; Viable counts; Organoleptic
Consumers are increasingly interested in their personal health, and expect foods to be safe and healthy. Such functional foods or neutraceuticals are of great demand in present time. The growing scientific evidence suggests that food supplements containing beneficial bacteria can provide an array of health benefits to the host. One such group of bacteria is commonly known as Probiotic bacteria. The realisation of importance of gut microflora in health restoration and maintenance kindled the interest in probiotics. Probiotics is defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [1]. Beneficial effects related to probiotics include antagonistic effects, competition, enhancement of digestion, strengthening of the immune system and stimulation of vitamin production [2]. At present, probiotic bacteria are widely used in human and animal nutrition because they beneficially influence the balance of the intestinal flora of the host. Probiotic therapy has been used to treat several gastrointestinal disorders, delay development of allergies in children, and also treat and prevent vaginal and urinary infections in women. The information on the recommend minimum concentrations of probiotic bacteria for effective function is still insufficient. However, adequate numbers of viable cells, namely the “therapeutic minimum” needs to be consumed regularly for the “probiotic” effect. Shah [3] has suggested a minimum viable number of 106 CFU ml-1 or gram, but recommends 108 CFU g-1 to compensate for reduction while passing through the gut. Controlled trials have shown that Lactobacillus GG can shorten the course of infectious diarrhea in children. Studies have also documented other health benefits relating to bioavailability of the nutrients, alleviation of lactose intolerance and maintenance of intestinal homeostasis [4].
Fermentation is an effective method of preservation, and contributes to the digestibility and the nutritive value of the final product [5]. The origin of cultured dairy products dates back to the dawn of civilization, and it has been mentioned in Bible and the sacred books of Hinduism [6]. But the scientific interest in this area was boosted after the work of Metchinkoff in 1908. Among the bacteria associated with food fermentation, lactic acid bacteria (LAB) is of predominantly important. LAB has contributed to the increased volume of fermented foods worldwide, especially in foods containing probiotics or health promoting bacteria. The fundamental reason for the development and acceptance of fermented foods can be ascribed to several safety and functional benefits. According to Steinkrause [7], the traditional fermentation of food serves several functions, i) enrichment of diet through the development of diversity of flavours, aroma, texture in food substrates, ii) preservation of substantial amounts of foods through lactic acid, acetic acid, alcoholic and alkaline fermentations, iii) enrichment of food substrates biologically with protein, essential amino acids, essential fatty acids and vitamins, and iv) detoxification during fermentation processing and decrease in cooking time, and thus, reduced fuel requirements.
It has been reported that chance contamination, favourable environmental and climatic conditions and serendipity, together played a role in the development of many of the cultured dairy products [8]. The Indian economy is agriculture based since the Vedic civilization. Cow and land were the major sources of economy. Fermented milk and milk products are, thus, intimately associated with Indian society. Indians have been consuming dahi or curd for centuries as a part of their traditional diets. In India, curd is essentially a vegetarian preparation. Dairy milk is used for curd preparation. Milk is boiled and is brought to temperature around 40°C; then an inoculum, as starter culture is introduced. The starter culture mainly composed of yeast or bacteria, or in combination. The preparation is then kept at room temperature (25-35°C), for 3-5 hours in summer and overnight during winter. The mode of preparation is found same as yoghurt. Curd is known for its characteristic taste and consistency. The word Dahi is derived from Sanskrit word Dadhi. Dadhi is one of the five elixirs (Panchamrita), often used in Hindu rituals [4]. However, the quality of dahi may vary with the type of starter culture used. The use of wild type of starter culture gives rise to poor grade dahi having less shelf-life and less effective. Therefore, a need was felt to formulate a probiotic curd (using the monoculture of the Probiotic strain Pediococcus spp GS4), with an extended shelf life by manipulating processing conditions to reduce the moisture content of curd, and also maintain the minimum viable cell population of the probiotic microorganism to attain the desirable health benefits. A practical approach to develop a Probiotic curd with a potential probiotic strain for extended shelf life and health benefit was the primary aim of the current study.
Standardisation of inoculum size for curd preparation
Pediococcus spp GS4 strain (NCBI Genbank: HM044322) isolated from Indian fermented food Khadi (Delicacy of Gujarat), with probiotic potentials [9], anti oxidative [10] and conjugated linoleic acid (CLA) producing [11], was used as a starter culture for curd preparation (Figure 1). Sterile MRS (de Mann Rogosa Sharpe) broth with final pH 6.5 ± 0.2 (at 25°C ) was inoculated with overnight grown Pediococcus spp GS4 culture (1% v/v), and incubated for 18 hours at 37°C. The viable count of the overnight grown culture was enumerated by serial dilution method.
Preparation of curd
Fifteen grams of skim milk powder (Everyday, India) was aseptically added to 60 ml of sterile water and mixed. The contents were placed in a water bath set to 70°C for 15-20 minutes to dissolve the contents thoroughly. It was then inoculated with 1% v/v of the inoculum containing the standard viable cells (108 CFU/ml). It was then incubated at 37°C for 18 hours, and observed for curdling. Enumeration of the viable cells in the curd was determined by serial dilution.
Physico-chemical analysis of the prepared curd
Moisture content of curd: Moisture content of curd was determined following the method described else where [12]. In brief, Pre-dried beaker was labeled and weighed accurately, and the mass was recorded. Small quantity of curd sample was placed and reweighed accurately. The moisture content was then evaporated by placing in Hot air oven at 90°C for 3 hours. The sample was then cooled and weighed. Percentage of moisture was calculated using the following formula:
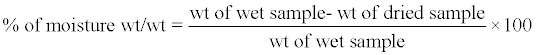 (1)
(1)
Estimation of total free amino acids: The free amino acids present in the curd sample were estimated colorimetrically at 570 nm using ninhydrin reagent [13]. Ninhydrin is a powerful oxidizing agent, which decarboxylates the α–amino acids to yield a bluish purple coloured product. The concentration of free amino acids in the curd sample was determined from the standard graph plotted with the water soluble amino acid, Leucine (1 mg/ml) as the reference standard.
Protein estimation: The total protein content in the curd sample was estimated colormetrically at 660 nm using bovine serum albumin (BSA) as the protein reference standard [14]. The concentration of protein in the curd sample was calculated from the standard graph using BSA as standard.
Estimation of total carbohydrates: The concentration of the total carbohydrates present in the curd sample was determined by phenol sulphuric method colorimetrically at 490 nm, using glucose as reference standard. The concentration of the total carbohydrate was estimated from the standard graph as glucose equivalent.
Determination of free fatty acid value: Five grams of curd sample was weighed accurately and placed in 250 ml Erlmnyer flask [12]. To the sample, 100 ml of neutralized ethanol and 2 ml of phenolphthalein indicator was added and titrated against 0.1 N NaOH. The end point of titration was indicated by a slight change to pink colour that persisted for 30 seconds. The volume of the titrant at the end point of titration was used to calculate the free fatty acid (FFA) value, using the formula given below:
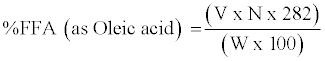 (2)
(2)
Where %FFA=percent free fatty acid in g/100 g expressed as oleic acid equivalent
V=Volume of titrant in ml (NaOH)
N=Normality of NaOH titrant (mol/1000 ml)
282=Molecular weight of oleic acid (g/mol)
W=sample mass (grams)
Formulation of curd into powder form for extended shelf life: The curd sample was formulated into powder form by subjecting samples to freeze drying (Lyophilization). The powdered curd was then evaluated for the shelf life by enumeration of viable count of Pediococcus spp GS4.
Evaluation of the shelf life of the lyophilised probiotic curd: The viable cell count of the lyophilized samples, stored at room temperature (28°C), and refrigerated temperature (10°C) was performed at two day intervals from 0 day (day of lyophilisation). 1 gram each of powdered curd stored was serially diluted using sterile normal saline (0.84% sodium chloride), and a 100 µl was plated on MRS agar and incubated at 37°C for 24 h. The cells were then enumerated accordingly.
Confirmation of the probiotic potential of Pediococcus spp GS4 after lyophilization: 1 g of the powdered curd was serially diluted to an appropriate dilution and plated onto MRS agar. The single colony was selected and inoculated into MRS broth for testing probiotic properties. The probiotic potential evaluated included: acid tolerance, bile tolerance, quantification of Lactic acid production and antimicrobial production.
Acid tolerance: Acid tolerance capability of the isolate was confirmed by viable count method [15]. One ml of GS4 grown in MRS broth for three generations having an optical density of 0.280 (at 660 nm) was inoculated into 9 ml of sterile MRS broth, with the pH adjusted to 3.5 with 1 N HCl, and was incubated at 37°C for 4 hours. At 0th hour and 4th hour, 1 ml of the sample was taken for enumeration by serial dilution using sterile normal saline (0.84% NaCl). A 100 µl of the appropriate dilution was plated onto MRS agar and incubated for 24-48 hours at 37°C. Colonies were enumerated using a colony counter. The reduction in log value after exposure to pH stress as compared to control was calculated to evaluate the survivability in the acid tolerance test using the formula: % survivability= (log CFU 4th hour/log CFU 0th hour)×100
Bile salt tolerance: Survivability on exposure to bile stress was evaluated by viable count method [15]. Like acid tolerance test, 1 ml of GS4 isolate was inoculated into 9 ml of MRS broth supplemented with bile salt (0.3% sodium thioglycollate), and incubated for 24 hours at 37°C. At 0th hour and 24th hour, 1 ml of the sample was taken, serially diluted, and 100 µl of the appropriate dilution was plated onto MRS agar. The plates were incubated at 37°C for 24 hours. The viable cell count was carried out using a colony counter, and the percentage survivability in the presence of 0.3% bile salt was calculated using the formula:
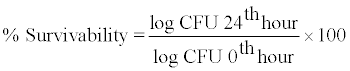 (3)
(3)
Determination of lactic acid production: The 24 h spent broth of the strain GS4 was collected by centrifuging at 10,000 rpm for 15 minutes at 4°C, and used for the quantitative estimation of the organic acid present. Few drops of phenolphthalein were added as an indicator to 20 ml of spent broth in the conical flask, and titrated against 0.1N NaOH. It has been standardised that one ml of 0.1 N NaOH is equivalent to 90.08 mg of lactic acid [16]. Amount of lactic acid produced was calculated from the standards provided. The experiment repeated and the average value was calculated.
Antimicrobial potential: The antimicrobial activity of the cell free extract (CFE) of the GS4 (10,000 rpm for 5 min at 4°C) was investigated. Neutralised CFEs were evaluated against the reference strains of Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 25619), and Listeria monocytogenes (ATCC 15313), by agar well diffusion method [17]. Inocula of reference strains from log phase (0.450-0.500 at 650 nm) were seeded onto Muller Hilton agar (pH 6.5 ± 0.2 at 25°C), using sterile swab. Wells were made using incinerated metal agar borer (8 mm). A 100 µl CFE was aseptically transferred to the wells along with ampicillin (10 µg/100 µl) and Nisin (100 µg/100 µl) as positive control, and negative control with un-inoculated sterile broth. The ZOI were observed after 24 hours of incubation at 37°C. The experiment was repeated thrice. The diameters of the ZOI were measured in mm and the average diameters were computed and compared with controls [18].
Organoleptic evaluation: The reconstituted curd sample (powdered curd mixed with appropriate quantity of water) was evaluated by ranking method for sensory characteristics, and overall acceptability by a random population of varying age groups selected from Vellore Institute of Technology University campus, Vellore.
The information on the recommend minimum concentrations of probiotic bacteria for effective function varies from study to study. The recommended “therapeutic minimum” needs to be consumed regularly for the “probiotic” effect. The study shows the survivability of Pediococcus spp GS4 strain qualified the “therapeutic minimum” [3]. The viable cells in the inoculum used for curd preparation was determined to be 1.24×109 CFU/ml, while the viable count of Pediococcus spp GS4 was calculated to be 2.46×109 CFU/ml of curd. This satisfies the minimum number of viable cells of probiotic microorganism in food carrier [3].
The prepared curd showed satisfactory physico-chemical parameters (Table 1). The study also qualifies the restoration of probiotic property after lyophilization. Acid tolerance is important not only for withstanding gastric stresses, but is also a prerequisite for their use as dietary adjuncts. It enables the strain to survive longer periods in high acid carrier foods, without reduction in their numbers [19]. Table 2 shows the results of the acid tolerance test. The Pediococcus spp. GS4 strain exhibited a survivability of 88.01%, when subjected to acidic pH of 3.5 for duration of four hours. Bile salt tolerance is also an important factor that affects the viability of LAB cells [20]. Bile plays a fundamental role in specific and non-specific defense mechanisms of the gut. Havennar et al. [21] has pointed out the tolerance to bile salt is a prerequisite for colonization and metabolic activity of the probiotic bacteria in the small intestine of the host. Table 3 shows the results of the bile salt tolerance potential of the isolate. In this study, the strain exhibited good bile tolerance potential with an increase in log value, and hence, a potential probiotic candidate.
| Physico-chemical parameters of prepared curd | ||
|---|---|---|
| Moisture content | 90.36% | |
| Ash content | 9.64% | |
| Total protein | 460 mg/ml | |
| Total reducing sugar | 0.86 mg/ml | |
| Free fatty acid | 67.7 mg/g | |
Table 1: Physico-chemical parameters of prepared curd.
| Strain | 0 hours | 4 hours | Survivability % | ||
|---|---|---|---|---|---|
| CFU/ml | Log CFU/ml | CFU/ml | Log CFU/ml | ||
| Pediococcus spp GS4 | 1.36×108 | 8.125 | 1.42×107 | 7.151 | 88.01 |
Table 2: Survivability in condition of acidic pH.
| Strain | 0 hours | 24 hours | Survivability % | ||
|---|---|---|---|---|---|
| CFU/ml | Log CFU/ml | CFU/ml | Log CFU/ml | ||
| Pediococcus spp GS4 | 1.59×108 | 8.20 | 1.95×109 | 9.29 | 113.3% |
Table 3: Survivability in condition of 0.3% bile salt (Sodium thioglycollate).
The concentration of lactic acid was found to be 2.43 ± 0.01 g/20 ml of the supernatant. Antimicrobial potential of LAB displays a wide range of antimicrobial activities. Among these, the production of lactic acid is of primary importance. Bhatia et al. [22] has also proposed that the production of organic acid by LAB is responsible for the inhibition of gastrointestinal pathogens. The ability to inhibit the growth of harmful bacteria is also considered as a desirable feature of probiotic bacteria [23]. The results of antimicrobial potential showed the maximum antimicrobial activity against S. aureus, with the average zone of inhibition of 13.9 ± 0.32 mm, moderate with P. aeruginosa with the average zone of 12.2 ± 0.45, and minimal inhibitory activity was observed with E. coli and L. monocytogenes, with the average zones of 11.9 ± 0.25 and 10.6 ± 0.85 mm, respectively. The positive control ampicillin (10 µg/100 µl) showed average zones of 39.10 ± 1.05, 17.6 ± 0.58, 28.5 ± 0.98, 36.7 ± 0.00 mm, respectively for S. aureus, P. aeruginosa, E. coli, and L. monocytogenes, respectively. Positive control with Nisin gave zone of inhibition only against S. aureus (19.5 ± 0.71 mm). The inhibition of reference bacterial member by neutralized CEF has been demonstrated by the production of a cationic antimicrobial peptide of 9.57 kDa, and found to be better candidate than Nisin (unpublished data).
The result of the viable count to evaluate the survivability of the Pediococcus spp GS4 cells is shown in Table 4. The shelf life of the powdered curd when stored at room temperature (28°C) was determined to be 5 days, and a decline in cell number was observed beyond the 6th day of storage. The viable cell count dropped to a 107 CFU/ml on the 6th day, which is below the recommended dose (108 CFU/ml) for the desirable health benefits of the probiotic inoculum. However, the viable count showed viability of 108 CFU/ml after 6 days of storage in refrigerated conditions (10°C). Hence, it can be inferred that shelf life of powdered curd formulation stored at room temperature is about 5 days, and an extended shelf life of 6 days was observed when stored in refrigerated conditions.
| Duration of Storage (Days) | Viable cell count CFU/ml under Storage conditions | |
|---|---|---|
| Room temperature | Refrigerated temperature | |
| 0 (Day of Lyophilisation) | 2.11 × 108 | 3.27 × 108 |
| 2 | 1.21 × 108 | 3.01 × 108 |
| 4 | 1.01 × 108 | 2.58 × 108 |
| 6 | 6.30 × 107 | 1.00 × 108 |
Table 4: Viable counts of the powdered curd stored at room temperature (28°C) and refrigerated (10°C) conditions.
Organoleptic survey showed overall acceptance of the reconstituted curd, with reference to factors such as taste, colour, aroma and texture.
Analysis of published literature reveals the various health promoting benefits of probiotic bacteria in fermented foods. There is good evidence that specific strains of probiotics are safe for human use and able to confer some health benefits on the host, but such benefits cannot be extrapolated to other strains without experimentation. The health benefits for which probiotics can be applied include conditions such as gastrointestinal infections, certain bowel disorders, allergy and urogenital infections, which afflict a large portion of the world’s population. The application of probiotics to prevent and treat these disorders should be more widely considered by the medical community. In addition, there is emerging evidence to indicate that probiotics can be taken by otherwise healthy people as a means to prevent certain diseases and modulate host immunity.
Fermented foods occupy one third of the diet worldwide. Fermentation process reduces the toxicity of the food along with the increased digestibility of raw materials. Different fermented foods are prepared worldwide using different substrates. Curd is one of the popular traditional food in southern India, commonly consumed by the rural folk. The functional value of this fermented food was improved by using the probiotic strain Pediococcus spp GS4 as inoculum/starter culture. This probiotic strain has been found to exhibit the key probiotic potentials such as acid tolerance, bile tolerance, production of lactic acid, anti-oxidative, as well as antimicrobial potential [9,10] and CLA production [11]. The demonstration of these properties has also been confirmed in this study. Therefore, content of the curd is capable of playing vital role in the maintenance of probiotic property and shelf life [24].
Curd is a low cost food that satisfies the rural need, and is nutritionally recognized. However, the lack of adequate scientific knowledge on fermentation process, absence of control over the fermentation variables, poor hygienic practices and the non-availability of refrigeration facilities lead to the variability/poor shelf life of the fermented preparation. This study has fulfilled this objective by the use of lyophilization technique. This is a widely used preservation technique in making liquid suspensions to powder form through the process of sublimation. The prerequisite for the probiotic inoculum to have a viable count of 108 CFU/ml was considered as criteria for assessing the shelf-life of the product. The viable count showed a decline in cell numbers when stored at room temperature, and the count dropped below 108 CFU/ml after the 6th day of storage. However the viable counts of 108 CFU/ml was observed on the 6th day of storage in refrigerated condition. Another study with GS4 showed satisfactory viability in gelatin capsule [25]. Results of a pilot study have shown the possibility of reducing the moisture content of the curd, and also to maintain its shelf life for about 5 days when stored at room temperature. This pilot study can be scaled up for the large scale production of the ready to eat Bio yoghurt with extended shelf life, which would make technology come possible in rural India.
Authors thank to VIT-TBI and Villgro Student Innovation Programme, which has given funds to GS to carry out her MS and the probiotic project at Centre for Infectious Diseases and Control, VIT University. GS also acknowledges the sincere cooperation extended by all the participants of VIT University, who took part in the survey for the organoleptic evaluation of the product.