Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2024)Volume 13, Issue 4
Background: Continuous positive airway pressure is the most effective therapy for Obstructive Sleep Apnea (OSA) but long-term adherence remains a challenge. Reducing Inspiratory Positive Airway Pressure (IPAP) below Expiratory Positive Airway Pressure (EPAP) may improve comfort and potentially compliance. The goal of this quality control evaluation was to determine if reducing IPAP below EPAP using the V-Com™ device maintained efficacy as assessed by the residual Apnea Hypopnea Index (AHI), P90%/P95% pressure requirements, usage time and leak.
Methods: We reduced IPAP below EPAP by adding non-compensated resistance (V-Com™) to the AutoPAP (APAP) circuit in patients with uncomplicated OSA. Four consecutive nights of data were collected with and without the V̇- Com™. Objective parameters obtained from the APAP devices over these 8 days included AHI, usage time, leak, pressure settings, and P90%/P95% pressures.
Results: There were 62 (34 male) patients in phase 1 (17 React Health Luna II, 22 Phillips-Respironics DreamStation 2, 23 ResMed AirSense 10 or 11 devices) and 40 (22 male) patients in phase 2 (ResMed AirSense 10 or 11 devices). The mean (SD) AHI decreased from 2.15 (2.35)/hour without the V-Com™ compared to 1.79 (1.74)/hour with the V-Com™ and 1.21 (1.06) to 0.97 (0.82)/hour in phases 1 and 2 respectively.
Conclusions: Our results indicate that reducing IPAP below EPAP with the V-Com™ device during APAP use provided equivalent therapy and did not interfere with the algorithms of the APAP devices. In addition, the V-Com™ device decreased the AHI, reduced leak and increased usage time without adversely affecting P90%/P95%pressures.
Inspiratory positive airway pressure; Expiratory positive airway pressure; Obstructive sleep apnea; V- Com™
In 1981, Sullivan et al., demonstrated that obstructive apneas could be abolished and sleep quality improved with Continuous Positive Airway Pressure (CPAP) delivered via a nasal mask [1]. Although these important observations were initially met with some scepticism [2], his findings were subsequently confirmed by other investigations and CPAP has become the enduring primary therapeutic modality for Obstructive Sleep Apnea syndrome (OSA) [3]. However, long-term adherence to therapy remains a challenge for many patients [4,5]. Common complaints include inconvenience, lack of perceived benefit, discomfort related to improper fitting of the interface, and difficulty expiring against positive pressure [6,7].
With the goal of improving adherence, innovations to mitigate the difficulty during exhalation have included Bi-level Positive Airway Pressure (BPAP) [8], and Expiratory Pressure Reduction Algorithms (EPRA) [9]. Early investigations of the pathogenesis of OSA emphasized the importance of negative airway pressure during inspiration with insufficient genioglossus activation [10,11]. Airway closure was thus considered to be principally an inspiratory phenomenon. Although increased airway resistance had been found during both expiration and inspiration [12], it was thought that EPAP might be safely reduced by simultaneously increasing IPAP.
In 1990, Sanders et al., introduced BPAP with higher IPAP and lower EPAP [8]. They found in 13 extremely obese patients (Mean BMI 57.41 ± 17.2 kg/m2) that IPAP alone could not treat apneas, but if sufficient EPAP was provided to prevent apneas, IPAP could be increased above EPAP to relieve hypopneas. These findings were subsequently confirmed by Resta et al., [13]. Based on previous work by Juhász et al., [14], Respironics, Inc., released an EPRA for CPAP devices called C-Flex™ in 2003. The concept of BPAP with IPAP>EPAP but with a smaller delta of about 1-3 cm H2O was developed for patient comfort and not grounded on any particular scientific advantage of which we are aware. Nevertheless, most standard CPAP devices currently have an EPRA option [9].
Unfortunately, measures to improve adherence by reducing EPAP and compensating by increasing IPAP have not clearly resulted in greater efficacy or compliance especially in uncomplicated non-obese patients [15-17]. Furthermore, reducing EPAP may compromise therapy while increasing IPAP may result in more adverse effects. Beginning with Reeves-Hoche et al., randomized controlled trials have not shown improved adherence and reduced side effects with BPAP to treat uncomplicated OSA [18].
Purposely reducing EPAP decreases End-Expiratory Lung Volume (EELV) which decreases the stability of the upper airway, reduces pharyngeal Cross-Sectional Area (CSApharynx), and increases Upper Airway Resistance (UAR) often yielding flow limitation, thus worsening OSA [19-21]. In 1992, Gugger et al., demonstrated these effects using rapid CT [19]. In 1994, Levy et al., had the same results using somnofluoroscopy [20]. In 1998, Series et al., found reducing EPAP below IPAP by even 1 cm H2O increased UAR and flow limitation [21].
Higher IPAP, when applied to the respiratory system, will seek out the most compliant segment of the respiratory system, which is the lungs. IPAP therefore cannot increase CSApharynx until lung volume increases enough to lower lung compliance down to the compliance of the pharyngeal walls (Cpharynx). However, as lung volume increases, Cpharynx decreases and pharyngeal walls become stiffer [22-25]. Increased IPAP only corrects hypopneas by increasing driving pressure and velocity over the obstructing site to compensate for the increased resistance.
If BPAP or EPRA improved adherence and reduced adverse effects, then this approach to therapy would have merit, but the evidence does not support this expectation [16-18]. In addition, the general belief, without evidence, in the need to maintain inspiratory pressure became most evident when mask compensation algorithms were added to PAP devices beginning in 2010. These algorithms further increase inspiratory pressure to compensate for varying resistance in different mask types. The belief in the need to maintain inspiratory pressure was so strong that these algorithms were apparently released without testing on patients.
Although many researchers and clinicians believe that maintaining high IPAP is the best treatment for hypopneas [26], there are no publications in which IPAP<EPAP has been tested for comfort or efficacy and no current bi-level PAP device can be set with IPAP<EPAP. We therefore hypothesized that we could intentionally reduce IPAP below EPAP, and maintain equivalent therapy, improve comfort, and potentially decrease adverse effects. The goal of this quality control evaluation was to determine if reducing IPAP below EPAP using the V-Com™ device maintained efficacy as assessed by the residual Apnea Hypopnea Index (AHI). We also quantified 90th or 95th percentile pressure requirements, usage time and leak with and without the V-Com™ device in place.
Physiologic background
Since no manufactured BPAP or CPAP device can reduce IPAP below EPAP, we reduced IPAP below EPAP by adding non- compensated resistance (V-Com™; Sleep Res, Murfreesboro, TN) to the device side of the exhaust port of the PAP circuit. The pressure drop across this resistor varies during inspiration and expiration according to the parabolic nature of turbulent flow (Figure 1).
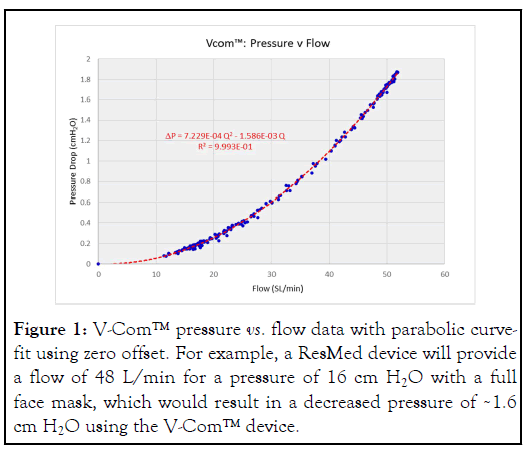
Figure 1: V-Com™ pressure vs. flow data with parabolic curvefit using zero offset. For example, a ResMed device will provide a flow of 48 L/min for a pressure of 16 cm H2O with a full face mask, which would result in a decreased pressure of ~1.6 cm H2O using the V-Com™ device.
The absolute flow from the CPAP device (VCPAP) is a function of the pressure setting and type of mask interface.
During the inspiratory phase of PAP, the circuit flow from the CPAP device (VCPAP) across the V-Com™ is high because it includes patient’s inspiratory flow (Vpatient), intended mask exhaust flow (Vexhaust), and unintentional leak flow (Vleak), the value reported as “leak” by the device manufacturers (Figure 2A).
The high flow across the V-Com™ during inspiration causes a flow-dependent pressure drop, thus reducing IPAP. During expiration, patient’s expiratory flow (Vpatient) exits directly through the exhaust valve (Vexhaust), although some could temporarily flow backwards toward the PAP device during high peak exhalation. Because Vexhaust is constant and based only on pressure, it becomes the sum of the reduced VCPAP and expiratory patient flow (Vpatient). Consequently, the circuit flow from the device across the V-Com™ is greatly reduced during expiration, resulting in negligible pressure drop (Figure 2B).
As a result of these effects, EPAP is preserved while IPAP is decreased.
Study participants
Clinically stable patients were sequentially recruited from a large community-based sleep medicine practice (Sleep Centres of Middle Tennessee [SCMT], Murfreesboro, Tennessee) between 1 July 2022 and 6 August 2022 (Phase 1). This group consisted of patients using devices manufactured by ResMed, Phillips-Respironics and React Health. Because of the unique situation in the USA where one manufacturer (ResMed) currently has over 80% of the market share, we elected to recruit an additional 40 patients (Phase 2) using only ResMed APAP devices (between 24 August 2022 and 14 September 2022). Inclusion criteria for both groups included: Excellent adherence to CPAP therapy defined as average usage ≥ 6 hours/night over the preceding 3 months, no therapy-related complaints, age ≥ 18 years, and use of an Auto-titrating PAP (APAP) device. Patients with any acute or unstable medical condition were excluded.
The V-Com™ device completed all required pre-market testing and was then registered with the Food and Drug Administration for market release in May 2022. All patients were provided written informed consent for participation. The study protocol was reviewed by the Institutional Review Board of the University of Utah and determined to be exempt from oversight. All patients provided written informed consent for participation.
Study protocol
In Phase 1, we obtained data from patients using one of the three manufacturers’ devices: Luna II (React Health, Sarasota, FL), Air Sense 10 or 11 (Res Med Inc., San Diego, CA), and Dream Station 2 (Philips, Inc., Pittsburgh, PA). In Phase 2, beginning one month after Phase 1, we recruited additional patients using only ResMed devices (Air Sense 10 or 11) according to the same selection criteria.
In both phases, patients had their APAP minimal pressure set 2 cm H2O below their P90% pressure (for Philips devices) or P95% (for ResMed and React Health devices) and their APAP maximum pressure set 4 cm H2O above their P90%/P95% pressure, which is defined as the pressure at which the device remains at or below for 90% or 95% of the time of an APAP therapy session. The P90%/P95% pressures are determined by the specific manufacturer’s algorithm which are proprietary but are generally driven to minimize detectable apneas and hypopneas or the (AHI).
Four consecutive nights of data were collected (without the V- Com™ device) via cloud-based software from each manufacturer: I-Code Connect (React Health, Inc.), Air View (ResMed, Inc.), and Care Orchestrator (Philips, Inc.). Following those 4 nights, the V-Com™ device was inserted into the patient circuit between the PAP device and the exhaust port in the mask (Figure 2A and 2B), and 4 additional consecutive nights of data were collected for analysis. To be included in the analysis, data had to be present for all 8 nights of the study.
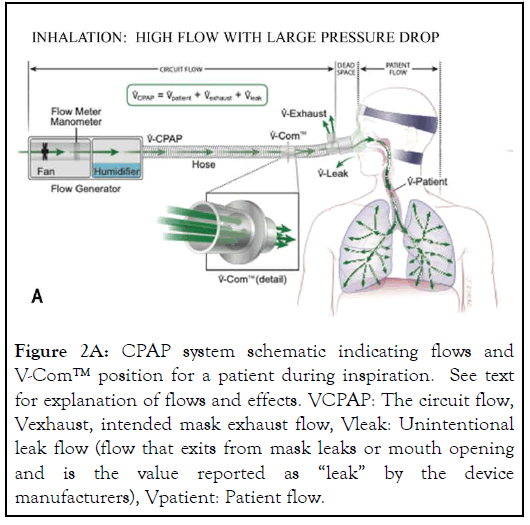
Figure 2A: CPAP system schematic indicating flows and V-Com™ position for a patient during inspiration. See text for explanation of flows and effects. VCPAP: The circuit flow, Vexhaust, intended mask exhaust flow, Vleak: Unintentional leak flow (flow that exits from mask leaks or mouth opening and is the value reported as “leak” by the device manufacturers), Vpatient: Patient flow.
Figure 2B: CPAP system schematic indicating flows and VCom ™ position for a patient during expiration. See text for explanation of flows and effects. VCPAP: The circuit flow, Vexhaust, intended mask exhaust flow, Vleak: Unintentional leak flow (flow that exits from mask leaks or mouth opening and is the value reported as “leak” by the device manufacturers), Vpatient: Patient flow.
The objective parameters obtained from the PAP devices over these 8 days included PAP device estimated AHI, usage time (hours per night), leak, PAP settings, and P90%/P95% pressures. These parameters are calculated differently depending on the manufacturer. However, we note that these algorithms are extensively used in sleep clinics to determine therapy efficacy, and are generally considered equivalent to one another. For the leak, the data were derived from the 95% leak for ResMed devices and the average leak for Philips devices. The data from the React Health Luna II devices had to be uploaded from a QR code by the patient and leak data were not available. These data were initially extracted from each cloud-based software by a research staff in the sleep medicine practice and then independently verified by a sleep technologist and sleep physician at the University of Utah Sleep/Wake Centre. Notably, Phase 2 obviated all this as data were computed only from one manufacturer’s algorithm.
Preference evaluation
Upon completion of the trial, participants were asked if they would prefer to continue using the V-Com™ device with their AutoPAP.
Statistics analysis
For the therapy equivalence evaluation, satisfactory efficacy was demonstrated if: 1.Titrated therapy pressures (P90%/P95%) were the same or less without and with the V-Com™ device; 2. The residual AHI was the same or less without and with the V- Com™ device; 3. Usage time was the same or greater without and with the V-Com™ device; and 4. Leak was the same or less without and with the V-Com™ device. To lessen the effect of night-to-night variability, the 4 nights of data with and without V- Com™ were averaged for each parameter for each patient.
Standard descriptive statistics of the population and outcome metrics included mean, SD, SE and range. Comparative statistics used a paired t-test as the data from the CPAP devices were all averaged over four-day periods. p-values<0.05 were considered significant. For all preference data, Wilson confidence intervals with an alpha of 0.05 were used.
In Phase 1, sixty-two patients (34 males/28 females) met the inclusion criteria, completed the study protocol, and had 8 nights of data available for analysis. Demographic characteristics and APAP device descriptions for these subjects are shown in Table 1.
| All | Male | Female | |
|---|---|---|---|
| N | 62 | 34 | 28 |
| Age (years) | 50.58 (11.06) | 48.38 (9.67) | 53.25 (12.19) |
| BMI (kg/m2) | 36.27 (7.18) | 36.04 (5.87) | 36.55 (8.61) |
| AHI (apneas + hypopneas/hour) | 34.01 (29.04) | 32.26 (28.20) | 36.13 (30.41) |
| React (N) | 17 | 9 | 8 |
| Phillips (N) | 22 | 12 | 10 |
| ResMed (N) | 23 | 13 | 10 |
| APAPMIN (cm H2O) | 8.3 (3.2) | 8.4 (3.2) | 8.3 (3.3) |
| APAPMAX (cm H2O) | 14.2 (2.8) | 14.9 (2.4) | 13.5 (3.2) |
Note: Values are reported as Mean (SD); N: Number of patients using a specific type of CPAP device (i.e., ResMed, Luna and Phillips-Respironics)
Table 1: Demographics and therapy settings (Phase 1).
The mean (SD) age, BMI and diagnostic AHI for the group measured 50.58 (11.06) years, 36.27 (7.18) kg/m2, and 34.01 (29.04)/hour, respectively. 17 were using React Health Luna II devices, 22 were using Phillips Respironics Dream Station 2, and 23 were using ResMed AirSense 10 or 11. The mean (SD) minimum and maximum APAP settings measured 8.3 (3.2) and 14.2 (2.8) respectively.
The effect of the V-Com™ device on the P90%/P95% pressure, residual AHI, leak and usage time are shown in Table 2.
| Parameter | Subjects | No V-Com | With V-Com | p value |
|---|---|---|---|---|
| P90 /P95 (cm H2O) |
62 | 11.27 (2.82) | 11.38 (3.01) | 0.136 |
| AHI (events/ hour) |
62 | 2.15 (2.35) | 1.79 (1.74) | 0.017 |
| Usage (hours) |
62 | 7.28 (1.32) | 7.56 (1.43) | 0.026 |
| Leak (L/ min) |
43* | 12.28 (9.32) | 8.12 (7.17) | <0.001 |
Note: Values are reported as mean (SD); *Leak measurement was not available in the React Health Luna II devices
Table 2: Effect of V-Com on the P90%/P95% pressure, residual AHI, usage time and leak (Phase 1).
There was no significant difference in the P90%/P95% pressure without and with the V̇-Com™ device in the circuit. The mean (SD) measured 11.27 (2.82) and 11.38 (3.01) respectively (p=0.136).
The frequency of respiratory events per hour was significantly reduced with lower IPAP using the V-Com™ device. The mean AHI (SD) obtained by the PAP device for all patients measured 2.15 (2.35)/hour without the V-Com™ device compared to 1.79 (1.74)/hour with the device (p=0.017).
Reducing IPAP with the V-Com™ device increased the mean (SD) usage time significantly from 7.28 (1.32) to 7.56 (1.43) hours (p=0.026). This 0.28 hour increased mean usage time translates to 16.8 minutes per participant. The mean (SD) leak recorded by both the Philips and ResMed devices decreased from 12.28 (9.32) to 8.12 (7.17) L/min (p<0.001).
In the second phase, all forty patients (22 males/18 females) recruited met the inclusion criteria, completed the study protocol, and had 8 nights of data available for analysis derived from the ResMed Air Sense 10 or 11. Demographic characteristics and APAP device descriptions for these subjects who were all using ResMed Air Sense 10 or 11 are shown in Table 3.
| All | Male | Female | |
|---|---|---|---|
| N | 40 | 22 | 18 |
| Age (years) | 51.75 (7.65) | 50.95 (7.47) | 52.72 (7.98) |
| BMI (kg/m2) | 37.70 (8.57) | 36.72 (8.47) | 38.9/38.89 (8.79) |
| AHI (apneas+hypopneas/hour) | 37.59 (27.98) | 39.02 (27.70) | 35.85 (29.02) |
| APAPMIN (cm H2O) | 9.2 (2.6) | 8.8 (2.5) | 11.5 (1.0) |
| APAPMAX (cm H2O) | 14.1 (2.5) | 14.0 (2.6) | 15.3 (1.2) |
Note: Values are reported as mean (SD)
Table 3. Demographics and therapy settings (Phase 2).
The mean (SD) age, BMI and diagnostic AHI for the group measured 51.75 (7.65) years, 37.70 (8.57) kg/m2, and 37.59 (27.98)/hour respectively. The mean (SD) minimum and maximum APAP settings measured 9.2 (2.6) and 14.1 (2.5) respectively.
The effect of the V-Com™ device did not result in clinically important adverse effects as shown in Table 4.
| Parameter | Subjects | No V-Com | With V-Com | p value |
|---|---|---|---|---|
| P95 (cm H2O) |
40 | 11.89 (2.15) | 12.10 (2.17) | 0.009 |
| AHI (events/hour) |
40 | 1.21 (1.06) | 0.97 (0.82) | 0.012 |
| Usage (hours) | 40 | 7.71 (1.35) | 7.82 (1.20) | 0.262 |
| Leak (L/min) |
40 | 8.84 (10.86) | 5.92 (6.37) | 0.007 |
Table 4. Effect of V-Com on the P95 pressure, residual AHI, usage time and leak (Phase 2).
The P95 increased slightly with the V-Com™ device in circuit (11.89 to 12.10 cm H2O). Note that the AHI actually decreased from 1.21 (1.06) to 0.97 (0.82)/hour and the leak was again significantly reduced from 8.84 (10.86) to 5.92 (6.37) L/min. 74% of all participants (76/102) preferred to continue using the V-Com™ device.
Our results indicate that reducing IPAP below EPAP with the V- Com™ device during APAP use provided equivalent therapy and did not interfere with the APAP algorithms of React Health, ResMed and Philips APAP devices. In addition, the V-Com™ device decreased the residual respiratory events (AHI) and increased usage time, which suggests the potential for improved therapy with reduced inspiratory pressures. The V-Com™ device also reduced the adverse effect of unintentional leak. We confirmed these results in a second trial utilizing only ResMed APAP devices.
Historically, airway closure has been considered by many to be principally an inspiratory phenomenon and therefore some have suggested that reducing IPAP could result in more residual respiratory events, particularly hypopneas. This historical view has led engineers, manufacturers, and much of the field to focus on maintaining inspiratory pressure despite the literature and physics suggesting otherwise. In this study, if hypopneas (or flow limitation) had emerged with a reduction of IPAP, we would have expected the APAP devices to respond by increasing the P90%/P95% pressure or the residual AHI to increase. However, neither occurred (Tables 2 and 4). Thus, the historically counterintuitive approach of purposely destabilizing the airway with lower EPAP and then increasing the pressure gradient across the obstruction (increased IPAP) failed to increase adherence [16,18], and potentially increased adverse effects [27,28].
This is the first study of reducing IPAP below EPAP with clear evidence that reducing IPAP decreases the adverse effect of unintentional leak. This reduction in leak was not unexpected since leak would be proportional to the peak and mean pressures in the system, and reducing IPAP reduces both peak and mean pressures in many PAP circuits. This decreased leak may be partly responsible for the reduction in residual AHI, either from reduced occurrence or reduced recognition of events. Reduced leak allows more accurate determination of patient flow and therefore more accurate event detection (and auto-titration response). To determine a respiratory event, which is a reduction in patient flow, the device must distinguish between circuit flow, patient flow, unintentional leak, and exhaust flows. By reducing unintentional leak flow, determination of patient flow is much simpler for the software in the device.
Seventy-four percent of long-term PAP users who were averaging >6 hours/night without complaints elected to continue use of the V-Com™ after their initial 4 nights during the trial. Besides improved comfort, other perceived benefits expressed by the participants included decreased machine noise, and improved daytime symptoms.
There have been multiple potential possibilities to explain why reduced IPAP is more comfortable. One is that reduced inspiratory pressure is more natural. Humans normally inspire with lower airway pressure. However, motor speed control on PAP devices attempts to prevent a drop in the circuit pressure during inspiration thereby forcing pressure and flow into the airway. The V-Com™ device likely counters some of this effect. Another explanation is expectation. IPAP pressure is not expected during a normal inspiration by the contracting diaphragm or the expanding lung. The more CPAP can feel like normal breathing, the more likely tolerance and perceived comfort will occur.
There are several limitations of this study. First, the therapy equivalence evaluation only included four nights under each condition, and longer periods may have yielded more reliable data. However, the primary purpose of the evaluation of long- term CPAP users was to verify therapy equivalence with the V- Com™ device as a quality assurance measure to ensure that the V-Com™ device did not interfere with event detection and the auto-titration algorithms of the three different manufactures’ devices. The V-Com™ device did not interfere with the operation of these algorithms and more than four nights would not likely have changed this outcome. The finding of increased usage time, although small, was not expected. However, to obtain meaningful adherence data will require a longer study.
Another limitation may have been the placebo effect. The long- term users were un-blinded and knew the V-Com™ device was being added to their circuit. The placebo effect could have clearly affected their comfort assessment and decisions to continue with the V-Com™. Finally, the assessment of AHI using the software of the various PAP devices with no measure of oxygen saturation or arousal is imperfect at best. Thus, further studies will need to include more accurate methods of AHI determination. However, as the same algorithms were used to assess AHI both with and without V-Com™, we believe these results strongly support a similar efficacy of PAP when the V-Com™ device is in place.
This first report of reducing IPAP to less than EPAP introduces a new concept for bi-level PAP therapy after 30 years. Mean airway pressure was reduced by lowering IPAP, which does not compromise therapy but yields a number of positive outcomes as outlined in this paper. Much more study is required, but reducing IPAP potentially provides an opportunity to improve PAP therapy for OSA and is certainly a paradigm shift for a well- established treatment with historically poor adherence.
The data utilized in this study were taken from CPAP device generated numbers with and without V-Com™. Thus, there are no raw tracings and only data-inclusive spread sheets. These spread sheets will be made available to interested investigators upon request with explanation of intended use.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Farney RJ, Hete B, White DP, Sundar KM, Lannom WD, Pucket MA, et al. (2024) Reducing Inspiratory Positive Airway Pressure (IPAP) to Treat Obstructive Sleep Apnea Provides Equivalent Therapy, Improves Comfort, and Reduces Unintentional Leak. J Sleep Disord Ther. 13:533.
Received: 09-Apr-2024, Manuscript No. JSDT-24-30712; Editor assigned: 11-Apr-2024, Pre QC No. JSDT-24-30712 (PQ); Reviewed: 25-Apr-2024, QC No. JSDT-24-30712; Revised: 02-May-2024, Manuscript No. JSDT-24-30712 (R); Published: 08-May-2024 , DOI: 10.35248/2167-0277.24.13.533
Copyright: © 2024 Farney RJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.