Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2016) Volume 4, Issue 2
The dispersal of toxic heavy metals by water from natural and anthropogenic is a worldwide environmental concern due to pollution. Despite some metals playing an important role in body, they are toxic when the level exceeds the tolerance limits while others such as lead have no known physiological value to human beings. Since heavy metals cannot be degraded, then their removal from drinking water is necessary. Mushrooms are readily available in Bomet County and their metal removal ability was investigated. The study aimed at removing heavy metals from water by adsorption using mushroom, as a cost-effective and sustainable method. The raw mushroom was modified with sodium hydroxide and characterization of both the parent material and its modified form was done using Fourier Transform Infrared spectrometry (FTIR). Sorption experiments were carried out using the batch adsorption method and sorption parameters including pH, contact time, adsorbent dose and initial metal ion concentration investigated. The results found out that the sorption capacity for cadmium ions ranged from 1.826- 25.285 mg/g by the unmodified edible mushroom (UEM), the modified edible mushroom (EM), unmodified toxic mushroom (UTM) and modified toxic mushroom (TM). For copper ions, sorption capacity ranged from 0.002-4.097 mg/g, while that of the lead ions ranged from 1.345-2.593 mg/g by the UEM, EM, UTM and TM respectively. The sorption capacity showed improvement on modification as sorption of cadmium increased from 1.826-25.285 mg/g by the UEM, EM, UTM and TM. At a pH range of 4-6, the sorbent material was found to remove up to 90% of the metals. The sorbent material had a removal efficiency of 95% of the metals in less than 20 minutes. The UEM and UTM fitted well in Langmuir adsorption isotherm model for cadmium and lead ions. For copper ions, UEM, EM, UTM and TM fitted in the Freundlich model. TM for lead ions best fitted in the Freundlich model. The bio-sorption kinetics was determined by fitting first-order-Lagergreg and Pseudo-second-order kinetics models to the experimental data. It was found that the data for lead was better described by the pseudo-second-order model. For copper ion, the data was best described by Ho’s pseudo second order for UEM and UTM, cadmium ions for all sorbents was best described by Lagergreg’s first-order kinetics. The FTIR analysis suggested the possibility of the participation of carboxyl groups in metal uptake. The levels of dissolved organic carbon (DOC) were found to be 19.0 mg/L in the raw material and 2.19 mg/L after modification. It was confirmed that modification minimized secondary pollution. This indicated that mushrooms have a potential application for the remediation of metal polluted waters.
Keywords: Mushrooms; Alkali modification; Sorption; Dissolved organic carbon
Water has a special property due to its ability to split ionic compounds it interacts with making it a universal solvent [1]. As such water in natural water bodies will have dissolved material of both organic and inorganic origin dispersed in this solvent. The presence of elevated concentrations of the dispersed solutes may render this commodity polluted and thus unsafe for human consumption. This implies that when water interacts with industrial, domestic, or agricultural wastes, it is a serious concern to human populations as well as the flora and fauna. This will result with the water being contaminated with a myriad of different components such as organic compounds, synthetic chemicals, nutrients, organic matter and heavy metals to the environment [2]. Interaction of such waters with the environment leads to loss of biodiversity due to pollution [2]. Anthropogenic activities such as metal processing industries and tanneries introduce heavy metals to water [3]. Unlike other organic waste heavy metals cannot be degraded biologically to products that are harmless and as such the only option is their removal from water. Irrespective of the fact that most heavy metals in the environment are in trace levels, continuous consumption of such water result to bioaccumulation of the same in vital organs and may pose a health risk [4-6]. Their toxicity depends on the type of metal, speciation and the type of organisms that are exposed to it. Examples of heavy metals include lead, zinc, copper, mercury, iron, manganese, cadmium, vanadium, antimony, selenium, arsenic and cobalt. Some metals such as lead interfere with normal body functions such as causing impaired brain development thus making consumers of lead contaminated waters to have challenges in life [7]. This makes heavy metals a concern to environmentalist, heath workers as well as industrial quality control managers [8-10]. Usually, these metals are leached into water from the land by rainwater and accumulated into ground water sources and other water bodies [11,12]. This renders the quality of the blue gold compromised and hence not suitable for consumption [13-16].
To make such water safe for domestic use, dissolved metal ions have to be removed. Removal has previously been achieved by the use of methods such as ion exchange, reverse osmosis and precipitation [17]. However such methods are expensive and not effective when the concentrations are in trace levels ranging from 1-20 μg.l-1 [18]. The use of biomass materials such as agricultural products which are abundant has been reported [19]. They, Abia et al. [19] reported that, waste cassava peels had a potential for the removal of trace metal ions from aqueous solutions over a wide range of concentrations. However, it has observed that all biomaterial had a challenge of leaching of organic matter during removal processes thus causing a ‘‘secondary pollution’’ [20]. Such biomaterials were not regeneratable and are susceptible to bacterial action [21,22].
This study therefore aimed to overcome such limitations by developing a cost-effective method by synthesizing an ion exchange biomaterial resin from both edible and toxic mushrooms [23-27]. Mushrooms are very nutritious as they have essential minerals and functional groups very important for life supporting enzymes and medicinal value [28]. This has given the mushrooms, despite growing very rapidly, an ability to contain some trace metals from the soil [29]. This ability was exploited in this study for the metal removal. The process was achieved by anchoring suitable functional groups capable of complexing metals on the biomaterial [30-36]. This would enable it to attract metals on its surface ions and remove them from water. The process was achieved by chemical modification of the mushrooms with a sodium hydroxide, a method exploited to prepare snuff from tobacco leaves to enable them is safe to chew, guard the tobacco leaves against degradation as well as pass the test of time [37]. The resulting solid material was then employed in its raw and modified forms for the removal of heavy metals in synthetic and contaminated waters. Its potential to minimize secondary pollution was also investigated [38-46].
Materials and reagents
All the solutions were prepared in double-distilled water, and the reagents were of analytical grade. Sodium hydroxide, sucrose, nitric acid and nitrates of lead, cadmium, and copper, were all supplied by Sigma Aldrich (Kobian, Nairobi Kenya). Mushrooms were obtained from a farm in Bomet County, Kenya.
Instrumentation
The FTIR spectrophotometer with an attenuated total reflectance (ATR) facility (Perkin Elmer 100 with sampling accessory-Waltham, MA, USA) was used to characterize the parent and the modified biomaterials [47-50]. The leached organic matter was determined using UV-Vis spectrophotometer (Specord 200-Germany). A flame atomic absorption spectrometry (FAAS; Buck Scientific 210 VGP) was used to determine the metal content in solution [51-54].
Experimental procedures
The sorbent used in this study was from both the edible and toxic mushroom collected from Sotik, Bomet County. They were cleaned on site to remove dirt, then with double distilled water. They then were transferred to the laboratory and dried in an air oven at 25°C until constant weight was attained [55-60]. The dried material was ground and sieved through a fine-mesh (150-250 mm). Some of the resulting material was modified, characterized and applied in the sorption process while the other was used its raw form. The sorption mechanism for both raw sorbent and its modification form were then investigated.
Modification of both edible and toxic mushroom
The modification process involved treating a solid sample of the dried mushroom (10.0 g) previously ground material with 0.1 M sodium hydroxide [61-64]. The resulting mixture was refluxed at a temperature of 80°C for three hours. The treated biomaterial was then washed with 100 ml of distilled water to bring the pH to neutral with dilute nitric acid. The solution was filtered through a sintered glass crucible and dried in a vacuum [64]. The resultant solid was dried in an oven at temperature of 80°C for two hours and then extent of modification was confirmed with FTIR and the product used for sorption experiments.
Characterization using FTIR
Analysis of the parent and the modified forms was done using FTIR spectrophotometer (FTIR-8400). The FTIR spectra were analyzed, and the functional groups in each were confirmed.
Batch sorption experiments
Sorption studies were carried out on a Lab-line mechanical reciprocating shaker model SSL2 (Harrogate, UK) using 100 ml screw cap plastic bottles. Known weights (0.3 g) of the adsorbent were in each bottle containing a known (40 ml) concentration of the metal ion of interest and the pH of the mixture adjusted to values between pH 3 and pH 7 (desired values) using 0.1 M nitric (V) acid and 0.1 M sodium hydroxide drop wise. The mixtures were then left to equilibrate allowing sufficient time for adsorption. Each of the resulting mixture was then filtered through Whatman No. 42 filter paper and the metal ions in the filtrate were determined by FAAS.
Optimization of pH
The effect of pH on the equilibrium was investigated by mixing 0.02g of sorbents material with 40ml of the test solution of 20 mg.l-1 concentration buffered with 0.1 mol.l-1 sodium acetate, and at pH values (3-7) by the addition of 0.1 M nitric acid drop wise. The mixture was equilibrated in a plastic screw cap bottles (100 ml) for 1 hour. The mixture was then filtered and the final concentration of the metal ions determined to establish the optimum pH for sorption of each respective metal.
Effect of the contact time on sorption of the metal ions
The effect of time on adsorption of metals by both the edible and toxic (modified and unmodified) mushroom was determined by mixing 0.3 g sorbent material with 40 ml of the metal solution whose concentration was 20 mg.l-1. The initial pH of the model solutions was adjusted to optimal value for each metal [65-68]. The respective mixtures were then allowed to equilibrate at predetermined time intervals of 2-100 minutes in a water bath shaker at 25°C. The sorbent was filtered using Whatman No. 42 and the metal ion concentration in the filtrate determined using FAAS.
Effect of metal ion concentration on sorption
The adsorption capacity was determined by mixing 0.3 g with 40 ml model metal solution (concentration 2-200 mg.l-1) at their optimum pH values with modified and unmodified sorbents. The mixtures were agitated for 1 hour, filtered and the concentration of the metal ions in the filtrate determined by FAAS.
Effect of adsorbent dose on percent recovery of metals ions
In the determination of adsorption capacity of the adsorbent for lead ions, 40 ml of the working solution of ions containing 20 mg.l-1 of metal ions into different plastic screw cap bottles and known masses (0.1 g, 0.5 g, 1.0 g, 1.5 g, 2.0 g and 2.5 g) of the adsorbent was added to the solution (specifically at the same and controlled initial pH of solution fixed at 7.0). The respective mixtures were agitated for 1 hour. The same procedure was followed in the determination of the adsorbent for cadmium ions and for copper ions. Content of the metal ions in the solution was then analyzed using FAAS [69-72].
Calculation of metal ion adsorption of modified and unmodified mushrooms
The amounts of metal ions adsorbed per unit mass by the modified and unmodified mushrooms during the batch investigation were calculated as amount adsorbed as given by equation 1 below
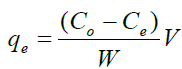 (1)
(1)
Where Co and Ce are the initial and equilibrium concentration (mg.l-1) of metal ions in solution, respectively, V is the volume of the solution in (ml) while W is the mass of the adsorbent in (g).
Analysis of environmental water samples
River water samples were collected from Nyangores River (Bomet County, Kenya). Known concentration of copper, cadmium and lead ions were spiked separately in to each of the samples (10 ml). The samples were loaded on to the solid-phase extraction (SPE) column containing 0.3 g of each adsorbent. The retained metal ions were then stripped with 5 mL of 0.5 M nitric acid and their concentration determined by FAAS.
Determination of secondary pollution in the treated water
The determination of leached organic matter was performed using dichromate method according to the procedure proposed by Baumann [73] and Gonzalez [74] by UV-Vis spectrophotometric method. Carbon standard solutions were prepared from sucrose in the range of 10–100 mg.l-1. Separate samples were prepared by placing a known weight of the respective biosorbents material (0.1 g) into a 250 ml round-bottomed flask containing 100 ml of water. Each of the mixture was placed on a magnetic stirrer and agitated for a period of 1 hour to extract soluble organic compounds. The solid was filtered off and the dissolved organic carbon content determined [75-82].
Characterization and optimization studies
FTIR characterization: The modified and unmodified adsorbents were characterized with FTIR and the resulting spectra are presented in Figures 1-4. The spectrum shows a band at 1225 cm-1 that may be attributed to the presence of organo sulphate groups [83]. This indicates the possibility of the presence of sulphur groups in the adsorbent. The band at approximately 930 cm-1 could be attributed to the C-H stretching [83]. There was also a broad band between 3921.0 cm-1 and 3710.8 cm-1 which had a very low sensitivity. The edible mushroom was modified and the results are presented in Figure 2.
The results show an enhanced broad band at 1878.5 cm-1 [84-86]. This could be a shift in the band at 1750.0 cm-1 in the unmodified material which was pronounced after modification. This band could be contributed by a keto group within the cellulistic structure [87]. Thus the main structure of the material could be a five membered ring [87]. The spectra also show a band at 1230.5 cm-1 that can be attributed to the presence of the amide functional group which is characteristic of charged NH4+ functional group [88]. It can also be due to stretching vibration of organic sulphates within the material [83,88]. This indicates the presence of sulphur in the adsorbent. The broad band at 1750 cm-1 could be as a result of a carbonyl, C=O, functional group [89-91]. The pattern in the peaks of both the raw and modified form is the same. The only difference is the enhancement of the broad band in the modified form which could be due to anchoring a keto group within the structure of the material. Both the raw and modified toxic mushroom materials were analysed and the results obtained were as presented in Figures 3 and 4 respectively. Figure 3 shows results upon characterization of the unmodified toxic mushroom species.
The spectra show a strong band at 3327.0 cm-1 attributed to either -OH or -NH groups [92,93]. But the band appears as two peaks, thus an indicator that it is most likely due to -NH groups [92,93]. The band at 2927.7 cm-1 was assigned to C-H stretching. The band at 1645.2 cm-1 may be due to the presence of the carbonyl group [81]. The band at 1317.3 cm-1 could be as a result of an amide or sulphamide group [89,90]. The band at 1155.3 cm-1 was assigned to the organic sulphates [83,94]. This indicates the presence of sulphur in the adsorbent. The material was modified and the results obtained presented in Figure 4.
The results show a strong band at 3398.3 cm-1 attributed to either -OH or -NH groups [92,93]. But the band appears as one peak, thus an indicator that it is most likely due to -NH group [92,93]. Treatment of raw biomass of mushroom with base improved the content of reactive centers such as carbonyl group of amide as shown by a sharp peak at 1652.9 cm-1 [95]. The transmitted peak at 2927.7 cm-1 present in unmodified toxic mushroom was shifted to 2925.8 cm-1 in modified toxic mushroom and the sharp peaks at 1651.0 cm-1 and 1558.4 cm-1 were broadened after modification [95]. The appearance of new peaks in modified toxic mushrooms at 1072.3 cm-1 and 1028.0 cm-1 may be due to C-O stretch or sulphonates [83].
The general observation from FTIR-characterization of the mushroom shown the presence of many functional groups such as – NH, C=O, -COOH, S=O, -OH and C-H which are capable of binding to metal ions [96-98].
Optimization of sorption parameters: Adsorption of metal ions is dependent on a number of factors. This study had to establish the conditions for removal of targeted metal ions from water. The parameters studied were effects of contact time, concentration of the initial metal ion, pH and dosage of the adsorbent.
Effect of pH on adsorption of metal ions: Adsorption of metal ions by sorbents is pH dependent as it influences the charge of the binding sites of the sorbent and the chemistry of the metal ion [99]. In this study, the optimum pH for metal ions uptake was determined through batch adsorption experiments. The optimum values were then applied in the subsequent experiments. The effect is due to the fact that, sorbents have sorption sites due to the presence of functional groups, whose charges are influenced by the pH [100]. At low pH, the adsorbent is positively charged because the pH is lower than the isoelectric point or point of zero charge (PZC thus pH < PZC). At such low pH range, adsorption is poor due to the charge on the adsorbent [101]. At high pH (PZC) the adsorbent is negatively charged contributing to a high adsorption [102]. This arises from the fact that when the metal is in solution, it is positively charged and will be attracted to the surface of the negatively charged adsorbent at that pH (pH > PZC), favouring adsorption. At pH > 6, there is metal hydrolysis, leading to precipitation due to formation of hydroxyl metal ion [103]. Results for the adsorption experiments at different pH values are presented in the Figure 5A.
Results show that maximum sorption of Cadmium ions by both unmodified and modified (edible and toxic) mushroom was observed at pH 4.0. This similar to what was reported by other researchers [21] who investigated sorption of metal ions by maize tassels. Mushroom both toxic and edible presents a high content of ionizable groups such as carboxyl, organic sulphates, and amino groups. Presence of these groups was confirmed by FTIR spectroscopic analysis (discussed in Section 4.1). At values lower than pH 4.0, cadmium ions removal was partially inhibited, due to the competition between hydrogen and cadmium ions on the sorption sites. At low pH, high hydrogen ion which restricts the metal cations to reach the sorption site, as in consequence of the repulsive force [104].
The highest bio-sorption efficiency of mushroom (edible and toxic) was observed at pH 4.2 with unmodified toxic mushroom (UTM) having high profile possibly due to the presence of carbonyl groups. Increase in cadmium ions removal, as the pH increased, can be explained in terms of point of zero charge (pzc) of the adsorbent and metal speciation taking place in the solution. The pH value, at which the charge of the solid surface is zero, is referred to as the point of zero charge (pzc). At pH value above pzc, there is a net negative charge on the biomass surface that promotes the uptake of metal ions. As the pH lowered, however, the overall surface charge on the biomass become positive which inhibit the approach of positively charge metal cations. It is likely that protons will then compete with metal ions thereby decreased interaction of metal ions with the sorbent as reported by Sag et al. [105]. The findings also compared well with by Padmavathy et al. [106]. According to Leyva, et al. [107] cadmium ions are the predominant ionic species at pH values below than 7.0 but precipitates above this pH value. As a result, pH optimization was carried at pH values below 7.0.
The results for the sorption of copper, obtained were presented. Figure 5B shows that the best sorption of copper ions by both the modified and unmodified (edible and toxic) mushrooms as a function of pH was observed to have a range between 4.5 and 5.0 for the modified toxic (TM) and unmodified edible (UEM) respectively and 5.8 for both unmodified toxic (UTM) and modified edible (UEM).
The effect of pH can be described in terms of pHpzc of the adsorbent and species of copper ions formed in the solution. When pH is equal to pHpzc, the surface charge of adsorbents is neutral and electrostatic attraction existing between the adsorbent surface and metal cations allows sorption. When pH is lower than pHpzc, the surface charge of adsorbents is positive, which inhibits the approach of positively charged cations. Rivera et al. [108] stated that an increase in the negative surface charge of the adsorbent that augments it to adsorb positively charged species. At pH greater than pHpzc, the surface charge of adsorbents is negative. Thus, cations adsorption on adsorbent is favorable at pH values greater than pHpzc while anion adsorption is favorable at pH value lower than pHpzc [109].
For sorption of lead ions (Figure 5C), was found to be strongly dependent on the pH of the solution. The optimum pH for the adsorption lead ions was about 5.0 for both modified and unmodified adsorbent. From the experimental data, sorption of lead by the unmodified sorbents (edible and toxic mushroom) had a wider sorption pH region (4.5–5.8) compared to the modified (pH 5.0), as shown in Figure 5C. This was attributed to the fact that modifications lowered the point of zero charge (pHpzc) which resulted in more negatively charged surfaces contributing to the observations made by Zhu, Yang, and Deng [110]. In addition, lead forms polynuclear complexes containing two or more lead atoms, in the presence of ligands at different pH conditions, favoring attachment of the metal ion onto the ligand [111]. At low pH (below 3), there was excessive protonation of the active sites at mushroom surface and this often refuses the formation of links between Pb2+ ions and the active site [103]. At moderate pH values (3-6), linked hydrogen ions being released from the active sites and adsorbed amount of metal ions is generally found to increase. At higher pH values (above 6), the precipitation is dominant or both ion exchange and aqueous metal hydroxide formation may become significant mechanisms in the metal removal process [104]. This condition is often not desirable as the metal precipitation could lead to a misunderstanding for the adsorption capacity. The pH value for maximum sorption for lead was observed as 5.1 closer to that reported by other researchers [112,113] who investigated the sorption of metals by marine algae. The pH value of 5.1 was adopted as the optimum value for lead sorption in our study.
Generally, observation from the experiments was that uptake capacities of the three heavy metals was pH dependent [90,91]. Study on the performances of the two species of mushrooms revealed that sorbent modification had a significant effect on the pH of maximum sorption of the metals analyzed [21]. The pH for the maximum adsorption was found to range between 4-6 for all the three metals. This implies that the adsorbent material is suitable for removal of metals from acidic water. Further experiments were carried out at optimal pH values for each respective metal ion.
Effect of contact time: The rate of metal ion adsorption is related to the efficiency of the adsorbent and activity of the metal and therefore controls the residence time of adsorbate at the solid–solution interface [114]. It is also related to the availability of the sorbents binding sites to hold the metal ions [114]. The results on the rate of adsorption are presented in Figure 6A.
The results show time dependent sorption of the selected metals. The rate of cadmium ions uptake in unmodified and modified edible mushroom was fast. The results show that 90% of the total uptake occurred within 20 minutes. The adsorption per gram of adsorbent was between 1.41 mg.g-1 and 1.86 mg.g-1. This was then followed by a slower uptake rate which resulted into a plateau after the sorption sites were occupied. The initial rapid rate may be due to a physical sorption or ion exchange at cell surface and the subsequent slower phase may be due to other mechanisms such as complexation, micro-precipitation, or saturation of the binding sites [114].
The sorption profile of copper (Figure 6B) show a rate of copper ions uptake of copper by all the adsorbent materials except UTM was 90% within the fast first 40 minutes. The latter removed 90% of the metal after 60 minutes. This was later followed by a slower uptake rate leading to a steady state where no significant adsorption occurred. Sorption by UTM attributed to the fact that modification lowered pHpzc which resulted in more negatively charged surfaces contributing to the observations made by Zhu, Yang, and Deng [110].
The rate of sorption of lead ions (Figure 6C) show that the rate of lead ions uptake by all the adsorbent materials was that 90% of the metal ions were removed from solution within the first 50-60 minutes. After that, the sorption remained constant after equilibrium was attained. This could be because the binding sites were limited and the remaining vacant surface sites were difficulty to be occupied by metal ions due to the formation of repulsive forces between metal ions on solid phase and the liquid phase [2]. The general observation was that the high rate of sorption was due to both chemisorptions and complexation while the slower phase may be due to saturation of the binding site and the subsequent attainment of equilibrium [115]. Mwangi et al. [21] investigated sorption of metals ions by maize tassels and reported that the sorption capacity of cadmium by the unmodified material was relatively lower (≈4 g.kg-1) than that for the modified (≈12.5 g.kg-1).
A similar profile was observed as reported by Mwangi et al. [21], of a general metal uptake were rather fast such that 90% of the total uptake occurred within 10 minutes, after which a slower uptake rate approached a steady state (where no significant sorption occurred). The initial rapid rate (within the first 10 min) may be due to a physical sorption or ion exchange at cell surface and the subsequent slower phase may be due to other mechanisms such as complexation, microprecipitation, or saturation of the binding sites. It was also observed that the modified sorbent had a better efficiency on the metal uptake.
Fazal and Rafique [116] investigated on mechanistic understanding of cadmium sorption by sulphonated and esterified spent black tea observed that for the fixed cadmium ions concentration and sorbent mass in the interface mixture, the sorption of metal increased as the contact time was increased. The sorption was, however, noted to occur in two phases of fast and slow rates. The biosorption rate was fast initially; about 50% of total cadmium was removed within 16 minutes. In the initial stages the high removal efficiency is due to the abundant availability of active binding sites on biomass, and with gradual occupancy of these sites, the sorption become less efficient in the later stages. The biosorption of cadmium reaches plateau value within 18 minutes. In this study beyond 20 min the cadmium ions desorption from porous sorbent shown increase. So the optimum agitating time for biosorption of cadmium was accepted as 20 minutes.
The results obtained from the study in rate of sorption of the three metals implied that 20, 40 and 60 minutes were sufficient time enough to achieve maximum adsorption for cadmium, copper and lead respectively.
Effect of sorbent dose on % removal of the metals ions: This was investigated by weighing varying masses (0.05-0.30g) of modified and unmodified mushroom (edible and toxic) and packed them in separate columns. Model solutions 40 ml, with a concentration of 20 mg.l-1 with their pH values adjusted to each metal’s optimum value were loaded onto the column at a flow rate of 3 ml minute-1. The content of the metal ions in the respective solutions were as presented in Figure 7A.
The results show that the percentage removal with Toxic mushrooms (TM) was highest with cadmium ions. This could have been attributed to the fact that the binding sites available in the modified toxic mushroom had high affinity for cadmium. The maximum percentage removal was 97.92% when the sorbent dose was 0.2 g. This could be because modification of raw sorbent with a base improves the content of reactive centers such as carbonyl group of amides hence relatively higher affinity for these metal ions in modified adsorbent [96]. Treating raw sorbent with sodium hydroxide increases the net negative charges on the surface of the sorbent thus possibly leading to the stronger electrostatic force of attraction between negatively charged surface of the adsorbent and positively charged sorbate ions hence efficiency towards improved removal [96]. Sorption of cadmium ions is dependent on the stability constant between the functional groups and the metal upon complexation. The higher that value, the higher the removal. Modification of the edible mushroom lowers the removal because of derivatization of the prior functional groups with a new one with a lower stability constant. This results to a lower adsorption of 95.8% from 96.5%. The unmodified toxic had a higher removal ability which is reduced upon modification due to the same reason of derivatization of the functional group.
The removal efficiency of copper ions as a function of sorbent dose was also investigated (Figure 7B). The results show that the adsorption of copper ions was that the UTM had the highest removal ability (74.7%) when the mass was above 0.3 g. The UEM had the least ability (65.7%) considering the same mass of adsorbent. This was due to the functional groups which bind metals during the sorption process. For the TM, adsorption was above 70% when the adsorbent dose was 0.3 g and 66.26% with adsorbent dose of 0.3 g for UTM and EM.
The percentage removal of lead with TM and EM (modified sorbents) was highest with 97.79% at 0.2 g and 96.24% at 0.25 g respectively for Pb2+ ions. This could have been attributed to the fact that the binding sites available in the modified (edible and toxic) mushroom had high affinity for lead ions. Also possibly because modification of raw sorbent with a base improves the content of reactive centers such as carbonyl group of amides hence relatively higher affinity for these metal ions in modified adsorbent [95]. However, maximum adsorption for UEM and UTM (unmodified sorbents) was lower with 95.3% for 0.15 g and 94.2% for 0.15g respectively (Figure 7C).
The general observation made for all the three metals was that, adsorption increased with increase in sorbent dose. This is because the more the sorbent, the more the site of adsorption hence more metal ions will occupy the available adsorption sites [117]. However, the increase of adsorbent against constant metal ions concentration leads to depletion of metal ions in solution hence a plateau. However, unmodified mushroom leached brown colour in the solution during the adsorption process unlike modified sorbent. This is a secondary pollution of water while being treated for the removal of metals. There is need to modify the raw mushroom before their use in the water treatment process, in order to prevent secondary pollution [118].
Effect of initial metal ion concentration (Determination of adsorption capacity): Metal adsorption is significantly influenced by the initial concentration of metal ions in aqueous solutions. In this study, the initial metal ion concentration was varied from 2-200 mg.l-1 maintaining the adsorbent dosage at 0.02 g. To obtain the sorption capacity of the bio sorbents, the data collected for each of the three metals was plotted as a function of concentration of the metal ions. The adsorbed metal ions were in mg.g-1 of the sorbent (qe) against the initial metal concentration in solution (Co). Figures 8A-8C shows results on the effect of initial metal ion concentration on the adsorption.
The Results show that, the maximum adsorption capacity for cadmium ions was found to be 0.47851 mg.g-1 on the UEM, 0.6804 mg.g-1 on EM, 0.3615 mg.g-1, for the UTM and 0.4755 mg.g-1 for the TM. The unmodified sorbent had a very low uptake as observed from the sorption plots. This confirms that the modification has a positive effect on sorption of the metals. The profile of sorption of cadmium was observed to increase with the increase in the initial metal concentration up to 150 mg.l-1 after which it levels off. An overall observation for the sorption of cadmium show a continuous metal uptake followed by a plateau as the concentration of the metal in the solution was increased.
Sorption of copper showed that the sorption of copper sorption on all the adsorbent increased with increase in concentration up to a maximum at initial metal ion after which a plateau was obtained. At that level, the adsorbent is said to be saturated hence the adsorption capacity [119]. The adsorption capacities of copper by the adsorbents were calculated using the Langmuir and Freundlich models.
The results for lead shows that, the rate of sorption by the adsorbent materials increased with increase in concentration up to a maxima at initial metal ion after which a plateau is attained due to be saturation of the active adsorbent sites [119]. From graph, it was found out that a plateau was leveling at a value of 0.36198 mg.g-1 for the UEM, 0.56966 mg.g-1 g for the EM, 0.58338 mg.g-1 for the UTM and 0.49292 mg.g-1 for the TM at maximum initial concentration of 150 mg.l-1. The enhancement of bio sorption capacity of lead ions with increasing initial concentration could be partially explained by higher availability of metal ions for bio-sorption.
Generally the profile of sorption of metal ions was observed to increase with an increase in the initial metal concentration in all the three metals up to 150 mg.l-1 after which a plateau was observed for all except for modified edible mushroom. The results also indicate that the metal ions uptake tends to saturate as the initial concentration is increased. This may be explained by a progressive increase in electrostatic mutual interaction between sites that have lower affinity for metal ions as the population of occupied sites increases [120]. A similar trend has been observed in removal of divalent metal ions copper, cadmium, zinc and lead ions by other bio [121-123].
It is also observed that sorption at low metal concentration was high as the sorbent to possess many sites with high affinity for the sorbate species [124]. An overall observation for all the three metals ions shows a continuous metal uptake followed by plateau as the concentration of the metal ion in the solution was increased. This may be due to an increase in metal ions for the fixed amount of the adsorbent material and due to saturation of available sites on the sorbent resulting in a saturation effect showing a steady state on the regression plot [119]. At this stage, the sorbent is said to have attained its operational maximum adsorption capacity.
The transmittance of certain peaks like those of sorption for cadmium, copper and lead ions using TM and sorption for lead ions using EM was observed substantially lower than unmodified. This may be an indication of a lesser degree of bond stretching due to change in chemical environment [125].
Adsorption isotherms: Adsorption isotherm models commonly describe the adsorption phenomena at the solid-liquid interface. They are essential data source for practical design of adsorption systems by determining factors such as adsorption capacity and understanding of the relation between adsorbent and adsorbate as well as the sorption mechanism [126]. Data from the Langmuir and Freundlich models were used to determine sorption capacity and mechanism of sorbentsorbate interaction in this study. This analytical method was important in the comparison of different adsorbents. The rate of sorption was established using the Lagergreg and Ho’s kinetic models.
Langmuir isotherms: Langmuir isotherm has been applied extensively for the sorption of many heavy metal ions. Langmuir assumes adsorption of an ideal gas on an ideal surface, that sorption occurs on a homogeneous surface; no interaction between adsorbed molecules and that adsorption can only occur at fixed sites and can only hold one adsorbate molecule at a time (monolayer). A further assumption is that no transmigration of adsorbed molecules on the adsorption surface [127]. Equation 2 shows the Langmuir equation.
 (2)
(2)
Where; qe is the amount of solute adsorbed (mg/g), Ce is the equilibrium concentration of the solute in the bulk solution (mg/L), Qmax is the monolayer adsorption capacity mg.g-1 and b is the constant related to the energy of adsorption (L/g). The experimental data were applied to the equation above and a plot of  against Ce ploted. A plot with a good linear correlation indicates that the Langmuir model describes the adsorption process [128,129]. Figure 9 below shows an example Langmuir plots for sorption of cadmium by UEM. Figure 10 below shows an example Langmuir plots for sorption of copper by TM.
against Ce ploted. A plot with a good linear correlation indicates that the Langmuir model describes the adsorption process [128,129]. Figure 9 below shows an example Langmuir plots for sorption of cadmium by UEM. Figure 10 below shows an example Langmuir plots for sorption of copper by TM.
Figures 9 and 10 shows Langmuir plots for sorption of Cd2+ by UEM with R2 = 0.8829 and that of Cu2+ by TM had R2 = 0.8782. The sorption of cadmium ions and copper ions with the same biosorbents gave an R2 value > 0.5 and slightly less than 0.9 indicating that the data has a strong correlation, hence the sorption mechanism can prescribe the Langmuir model. Whenever the R2 value is lower than 0.5, it can be concluded that the scatter had no correlation and that the adsorption cannot prescribe the Langmuir model and can probably be described by another model.
Freundlich isotherms: The Freundlich isotherm assumes a heterogeneous surface with non-uniform distribution of adsorption heat over the surface (binding sites are not equivalent) and/or a multilayer adsorption [130]. The mono-component Freundlich isotherm equation is given by equation 3 below.
 (3)
(3)
Where Kf is the Freundlich isotherm constant related to the sorption capacity, n is the constant related to affinity of metal ions on adsorbent, qe and Ce has the same meaning as that of Langmuir. By plotting log qe versus log Ce, the constant n and Kf can be determined from the slope and intercept, respectively. Good linear correlation implies that the sorption fits the Freundlich model. Figure 11 shows an example of Freundlich plots for sorption of cadmium UEM.
The results shows Freundlich plots for sorption of cadmium by UEM with R2 = 0.5337. This implies that sorption prescribes to a Freundlich model. Figure 12 below shows an example of Freundlich plots for sorption of copper TM.
The results shows Freundlich plots for sorption of copper by TM had R2 = 0.916. This implies that sorption prescribes to a Freundlich model. In both cases of copper and cadmium, it means that the interactions is multi-site adsorption for heterogenous surfaces expecially for highly interactive surfaces. The experimental data from the results extracted on the on the effect of sorption on concentration on sorption obtained upon treatment with both the Langmuir and Freundlich isotherms were recorded in a tabular form. Table 1 below shows the results obtained for adsorption isotherm of cadmium ions.
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Sample | Qmax mg/g | R2 | Kf mg/g | R2 | Best Model |
| UEM | 1.826 | 0.8829 | 3.3508 | 0.5337 | Langmuir |
| EM | 25.285 | 0.7082 | 29.7075 | 0.8296 | Freundlich |
| UTM | 2.3353 | 0.9693 | 4.3947 | 0.7117 | Langmuir |
| TM | 6.5901 | 0.0704 | 9.8404 | 0.9211 | Freundlich |
Table 1: Adsorption kinetics of cadmium ions.
Table 1 shows that for cadmium ions, EM and TM best fit Freundlich model with R2 = 0.8296 and 0.9211 respectively. UEM and UTM with R2 = 0.8829 and 0.9693 best fit the Langmuir model. The adsorption capacities (Qmax) of cadmium ions from Langmuir isotherm model was determined to be 1.826 mg/g for UEM, 25.285 mg/g for EM, 2.3353 mg/g for UTM and 6.5901 for TM. Freudlich constants were calculated for Cd2+ where the Kf value was found to be 3.3508 mg/g for UEM, 29.7075 for EM, 4.3947 for UTM and 9.8404 for TM.
Generally for cadmium ions, modified biosorbents had high adsorption capacity on fitting to both Langmuir and Freundlich isotherm models. Modification could possibly have led to stronger electrostatic forces of attraction between negatively charged surface of adsorbent and positively charged sorbate ions, hence efficiency towards removal improved. Table 2 shows the results obtained for adsorption isotherm of copper ions.
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Sample | Qmax mg/g | R2 | Kf mg/g | R2 | Best Model |
| UEM | 4.0978 | 0.0312 | 24.7667 | 0.8792 | Freundlich |
| EM | 2.2136 | 0.4773 | 25.5694 | 0.8553 | Freundlich |
| UTM | 1.7524 | 0.0386 | 19.45687 | 0.635 | Freundlich |
| TM | 0.0021 | 0.8782 | 26.0469 | 0.916 | Freundlich |
Table 2: Adsorption isotherm of copper ions.
Table 2 shows that for copper ions, UEM, EM, UTM and TM best fit Freundlich model with R2 = 0.8792, 0.8553, 0.635 and 0.916. Adsorption capacities (Qmax) was found to be 4.098 for UEM, 2.2136 for EM, 1.7524 for UTM and 0.0021 for TM. UEM was found to have highest adsorption capacity of 4.0978 mg/g. This is possibly because copper species involved are highly interactive with the sorbents [129]. Huang and Vansant also made similar observations and concluded that the electrostatic components, polarization energy and field-dipole energy were important contributors to the phenomenon. The sorption of copper, therefore, could be as a result of more than one mechanism and that a degree of heterogeneity is possible for ionic species involved in the solution and on the surface. Physical adsorption due to Van der Waals forces of attraction could also have contributed to the interaction phenomenon [131]. Table 3 shows the results obtained for adsorption isotherm of lead ions.
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Sample | Qmax mg/g | R2 | Kf mg/g | R2 | Best Model |
| UEM | 2.5936 | 0.991 | 3.6255 | 0.4702 | Langmui |
| M | 1.5536 | 0.8519 | 4.374 | 0.7765 | Langmuir |
| UTM | 1.7355 | 0.9175 | 3.9788 | 0.5568 | Langmuir |
| TM | 1.3458 | 0.7569 | 4.5557 | 0.8081 | Freundlich |
Table 3: Adsorption isotherm of lead ions.
(Qmax) of lead ions from Langmuir isotherm model was determined to be 2.5936 mg/g for UEM, 1.5536 mg/g for EM, 1.7355 mg/g for UTM and 1.3458 mg/g for TM. Kf from Freundlich model was determined to be 3.6255 mg/g for UEM, 4.3740 mg/g for EM, 0.5568 mg/g and 0.8081 for TM. Adsorption capacities (Qmax) for sorption of lead by unmodified biosorbents (both edible and toxic) was high with 2.5936 mg/g for UEM and 1.7355 mg/g for UTM compared with those for modified biosorbents.
Generally for the three metal ions, the adsorption capacity of cadmium ions was higher than that of lead and copper ions in the modified biosorbents. This may be because of the presence of sulfoxide group in the adsorbent which has a selective high affinity for cadmium ions than copper and lead ions [132].
Sorption kinetics
Kinetic studies: The rates of metal adsorption studies were analysed using Lagergreg first-order and Ho’s second-order kinetics [133,134]. This gave an insight into the number of molecules taking part in each step of the adsorption. The experimental data were fitted to both the Lagergreg first order and Ho’s second-order kinetic expression so as to investigate the molecularity of the adsorption mechanism and the ratecontrolling steps [133,134]. The Lagergreg first-order and Ho et al.’s second-order kinetic models are given in equations 4 and 5 respectively.
 (4)
(4)
 (5)
(5)
Where
Co is the adsorption per unit mass of adsorbent at equilibrium;
K is the adsorption rate constant;
A is the intercept;
Ct is the concentration at time t.
The experimental data for the three metals were then fitted to the Lagergreg pseudo first-order and Ho et al.’s pseudo second-order kinetic models.
The experimental data from the results on the effect of time on sorption obtained were treated with both the kinetics isotherms. The results obtained were as presented in the Tables 4-6. Table 4 below shows kinetics isotherm of cadmium ions with all the adsorbents.
| Lagergren pseudo first-order | Ho’s pseudo second-order | ||||
|---|---|---|---|---|---|
| Sample | R2 | K | R2 | K | Comments |
| UEM | 0.5982 | 0.0423 | 0.3585 | Sorption, first order | |
| EM | 0.5794 | 0.006 | 0.5503 | Sorption, first order | |
| UTM | 0.7839 | 0.0182 | 0.5955 | Sorption, first order | |
| TM | 0.9578 | 0.0151 | 0.8623 | Sorption, first order | |
Table 4: Sorption kinetics studies of cadmium ions.
| Lagergren pseudo first-order | Ho’s pseudo second-order | ||||
|---|---|---|---|---|---|
| Sample | R2 | K | R2 | K | Comments |
| UEM | 0.7044 | 0.7091 | 0.05 | Sorption, second- order | |
| EM | 0.6637 | 0.024 | 0.6635 | Sorption, first- order | |
| UTM | 0.8295 | 0.8306 | 0.06 | Sorption, second- order | |
| TM | 0.6281 | 0.04 | 0.6274 | Sorption, first order | |
Table 5: Sorption kinetics studies of copper ions.
| Lagergren pseudo first-order | Ho’s pseudo second-order | ||||
|---|---|---|---|---|---|
| Sample | R2 | K | R2 | K | Comments |
| UEM | 0.6752 | 0.6809 | 0.051 | Sorption, second- order | |
| EM | 0.9159 | 0.9162 | 0.06 | Sorption, second- order | |
| UTM | 0.7719 | 0.7727 | 0.056 | Sorption, second- order | |
| TM | 0.9096 | 0.9104 | 0.061 | Sorption, second- order | |
Table 6: Sorption kinetics studies of lead ions.
From Table 4, when the experimental data for cadmium was treated with the Lagergreg first-order kinetics and a plot of ln (C0–Ct) against time obtained, it gave a correlation coefficient value (R2) of 0.5882, 0.5794, 0.7839 and 0.9578 for UEM, EM, UTM and TM respectively which is high (expected value is 1.0), indicating that there was strong correlation in the scatter. But when treated with Ho’s second-order kinetics, the R2 value was lower than pseudo first order with 0.3585, 0.5503, 0.5955 and 0.8623 for UEM, EM, UTM and TM respectively. This indicated that the sorption of cadmium ions with all the sorbents was likely to be pseudo first order hence suggests that one mechanism controls the adsorption process. Table 5 shows kinetics isotherm of copper ions with UEM, EME, UTM and TM.
From Table 5, when the experimental data for copper was treated with both kinetics isotherms and R2 values were obtained for all the adsorbents, high value of R2 were obtained when the data were treated with Lagergreg pseudo first –order with modified adsorbents compared to unmodified sorbents which exhibited Ho’s pseudo second-order. This is possibly because upon modification only one functional group is available in the adsorbent hence demonstrating a single site reaction. For unmodified adsorbents, many functional groups were available hence more than one site available for interactions. The sorption of copper ions on UEM and UTM had higher R2 values of 0.7091 and 0.8306 respectively hence Ho’s pseudo second order, with Lagergreg pseudo first order had a lower value of 0.7044 and 0.6637 for UEM and UTM respectively. For modified sorbents EM and TM, R2 value was 0.6637 and 0.6281 respectively when treated with Lagergreg pseudo first order. When treated with Ho’s pseudo second order R2 was slightly lower with 0.6635 for EM and 0.6274 for TM. Table 6 shows kinetics isotherm of lead ions with UEM, EM, UTM and TM.
Table 6 above shows experimental data for lead ions where the two chemical reaction rate models used in fitting the experimental data gave an indication of the reaction pathway. From the two models, the pseudo second-order equation fits the experimental data better with a high correlation coefficient R2 with all the adsorbents. On the basis of this parameter fitting, the biosorption of Pb2+ onto the Agaricus biporus and Amanita phalloides is a second order reaction, and not a firstorder one. This is in agreement with the findings of Ghaedi et al. [135], who studied the biosorption of Pb2+ ions onto S. cerevisiae biomass R2 for UEM, EM, UTM and TM was 0.6809, 0.9162, 0.7727 and 0.9104 respectively with Ho’s pseudo second order. This is slightly higher than Lagergren pseudo second order with R2 value of 0.6752, 0.9159, 0.7719 and 0.9096 for UEM, EM, UTM and TM respectively.
Analysis of environmental water samples: The results of river samples analysis for the content of copper, lead and cadmium ions are given in the Figures 2-6 [27,28]. Figure 13 shows the profile of percentage removal on the effect of metal ion concentration on adsorption of cadmium using river water.
Figure 14 shows the profile of percentage removal on the effect of metal ion concentration on adsorption of copper using river water.
Generally, the results in Figures 10-12 show that there was high removal of metal ions at low concentration but decreased with an increase in the spiked metal ions concentration. This may be due to an increase of the metal ions against constant adsorbent material (Figure 15).
The sorption of lead by modified adsorbents was found to be the highest followed by copper and then cadmium. This adsorption trend may be explained by the fact that copper ions compared to lead ions, have relatively higher affinity for ligands containing a quaternary Natom in dissolved organic carbon (DOC) [136]. This would result in the removal of copper by the DOC and hence posing less competition for the lead ions to adsorb on the modified adsorbents [136]. Such ligands, which are smaller than the adsorbent, have a higher affinity for the metal due to their high basicity [137]. The above result shows that the method was effective and reliable as it achieved with recovery values similar to those obtained in model solutions.
Dissolved organic carbon: Absorbance readings to determine the DOC were taken at an optimized wavelength of 606 nm. A calibration curve was prepared whose linear equation was describing was: y = 0.0052 + 0.006 and regression R2 = 0.983. This closely agrees to one given by Lewis et al. [138] when he used a 10 mm cell, as they analyzed some Venezuelans and southeastern USA waters. The average DOC content for the modified material was 2.19 mg/L whereas the unmodified material had 19.0 mg/L. The results indicate that modification had a significant effect on minimizing leached organic matter in the treated water.
This study successfully functionalized the mushroom which was confirmed by FTIR analysis. It was confirmed that sorbent modification reduced the problem of leaching organic matter during the treatment process. The average DOC content leached by the modified material was 2.19 mg/L whereas the unmodified material had 19.0 mg/L. This implies that modification was alleviating the secondary pollution phenomena. Kinetic studies confirmed that sorption for lead was of pseudo-second-order but pseudo-first-order for both cadmium and copper. The sorption experiments also revealed that 90% of the metal ions were removed within the first 20 minutes of contact time. The adsorption capacity for cadmium ions was 1.826 mg/g by the unmodified edible mushroom (UEM), 25.285 mg/g by the modified edible mushroom (EM), 2.335 mg/g by the unmodified toxic mushroom (UTM) and 6.5901 mg/g by the modified toxic mushroom (TM). For copper ions, sorption capacity was found to be 4.097 mg/g, 2.213 mg/g, 1.752 mg/g 0.002 mg/g by the UEM, EM, UTM and TM respectively. The sorption for the lead ions was found to be 2.593 mg/g, 1.553 mg/g, 1.733 mg/g and 1.345 mg/g by the UEM, EM, UTM and TM respectively. The results show that the calculated sorption capacities obtained from the modification had a negative effect on sorption of the respective metals but there were numerous advantages such as stability and secondary pollution. It was also observed that the sorbent in the SPE column could be regenerated by stripping the attached metal ions with a dilute acid (1.0 molL-1 HNO3) thus the material could be regenerated when exhausted. In conclusion, our study has shown that the modified mushroom can be used as an effective sorbent for the removal of copper, cadmium, and lead ions in the remediation of contaminated water samples at sub-neutral pH range.