Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2019) Volume 10, Issue 1
With a resurgence of the nuclear industry’s fortunes, waste management will be an even greater consideration; reducing wastes, better segregation and treatment that lower the impact on waste storage facilities and repositories, in particular geological repositories will help the sustainability of the industry. We at the University of Central Lancashire (UCLan) proposed in a previous publication a sequential chromatographic separation process, Alternative Reprocessing Technology (ART) for Fission Products (FPs) and Minor Actinides (MAs) separation from spent fuel dissolver liquor, as an improvement to/replacement for PUREX (Plutonium Uranium Redox Extraction). This publication addresses the removal of one particular fission product, cesium, and its impact on waste management, and down-stream PUREX operations. Although our proposed process is still in its infancy, its impact on the PUREX process could be significant, with major gains in the separation circuit, waste management of High Level Waste (HLW) and subsequently waste disposal to and design of a geological repository. This paper briefly describes; our concept, some preliminary experimental data and why re-classification of the bulk of HLW (High Level Waste) to Intermediate Level Waste (ILW) would be possible.
Keywords: PUREX; Extractive chromatography; Separation; Fission products; Spent fuel reprocessing; Elimination; HLW; Waste repository
The PUREX process [1] has been the accepted separation technology for the reprocessing of spent nuclear fuel for more than 60 years. The merits and efficiency of this process have been unchallenged since it produces highly purified plutonium and uranium for recycling [2], but at a cost of producing comparatively large volumes of High Level Waste (HLW) [3].
The concept (ART) proposed in our previous publication (Advanced Reprocessing- The Potential for Continuous Chromatographic Separations), (Figure 1) [4,5], is based on the separation of fission products and minor actinides from uranium and plutonium isotopes in spent fuel dissolver liquor using continuous chromatographic-type separation [5]. Our process offers many advantages to the PUREX process as discussed later in this paper. Elements of our proposed process could be, in the interim, incorporated into the existing PUREX flowsheet, with the potential to improve efficiency, safety and economy, whilst reducing the volume of HLW. This would be a major improvement and one needed for the next generation (GEN 3) and subsequent generation of nuclear reactors. ART would encourage a greater proportion of spent fuel to be reprocessed compared with the current figure of less than 30%, thus adding to the sustainability of the nuclear fuel cycle. Non-proliferation concerns are pacified, as U and Pu, if needed, are not separated until the final stages of our technology.
The most energetic heat generating FPs for spent nuclear fuel that has been cooled for several years prior to reprocessing are cesium and strontium radionuclides, which are β/γ emitters. The removal of cesium and strontium from the spent fuel dissolver liquor and onto a solid-phase material would produce a down-stream liquor that is significantly less heat generating. Removal of these FPs and their associated radiation would allow more amenable downstream processes to be considered while providing a ready disposal route for the heat generating Cs and Sr radionuclides.
The challenges for the current PUREX process with these heats generating fission products are:
1. The radiolytic degradation of the PUREX extractant, tri-butyl phosphate, produces degradation products such as di-butyl hydrogen phosphate and mono-butyl di-hydrogen phosphate which remain in the solvent phase and have a greater affinity for fission products [6] (Box 1, Figure 2);
2. The solvent phase requires routine washing with alkali to remove these degradation products [7] (Box 2, Figure 2);
3. Fully degraded tri-butyl phosphate, i.e., phosphoric acid transfers from the solvent phase into the highly active aqueous phase, which ultimately results in the formation of cesium phosphomolybdate (CPM) during the evaporation of this aqueous stream, [8] (Box 3, Figure 2);
4. Formation of evaporator solids causes difficulties during transferring of evaporator product liquor to down-steam liquid storage, [9] (Box 4, Figure 2);
5. This highly active liquid requires continuous cooling, [9] (Box 5, Figure 2).
The attributes of ART i.e., removal of cesium isotopes from the spent fuel dissolver liquor would:
1. Reduce TBP degradation.
2. Eliminate the formation of CPM during evaporation and subsequent blockages of pipelines.
3. Possible elimination of liquid storage facilities post evaporation.
4. Direct vitrification of HLW from the high active chemical separation stage.
5. Much simplified plant and equipment requiring less shielding.
6. Significant capital cost savings
7. Contributes to the non-proliferation treaty.
Impact of cesium removal from spent fuel dissolver liquor on HLW management
The quantity of spent fuel produced annually from a 1000 MWe nuclear power plant with a burn up of about 45GWd/t HM is about 27 t oxide fuel/a i.e., about 25 t U/a [4]. On reprocessing this spent fuel with subsequent vitrification of the high level radioactive liquid produces about 110 litres/t U of classified solid waste, i.e., about 3,000 litres/a [4]; this would equate to about 3,000 M3 for a reprocessing plant with a 1,000 t U annual throughput. The bulk of heavy metals in spent fuel dissolver liquor comprises of uranium and plutonium isotopes, but these isotopes contribute little to the heat load; whereas cesium isotopes (Cs-134 and Cs-137) contribute 269 W/t U (~17%) (Table 1) With their removal, the daughter radionuclide Ba-137m would also disappear in a matter of minutes, producing a total loss of 705W/t U (~46%). This may produce waste of the intermediate level classification, i.e., residual heat load 825 W/t U.
| Radioisotope | Bq/t U | Heat rating factor (W/Bq) |
Heat rating contribution (W/tU) |
|---|---|---|---|
| Y-90 | 3.14E+15 | 1.50E-13 | 4.70E+02 |
| Sr-90 | 3.14E+15 | 3.13E-14 | 9.84E+01 |
| Rh-106 | 8.90E+13 | 2.60E-13 | 2.31E+01 |
| Sb-125 | 4.65E+13 | 8.54E-14 | 3.97E+00 |
| Cs-134 | 5.02E+14 | 2.75E-13 | 1.38E+02 |
| Cs-137 | 4.38E+15 | 2.98E-14 | 1.31E+02 |
| Ba-137m | 4.14E+15 | 1.06E-13 | 4.39E+02 |
| Ce-144 | 3.33E+13 | 1.78E-14 | 5.92E-01 |
| Pr-144 | 3.33E+13 | 1.98E-13 | 6.60E+00 |
| Eu-154 | 1.72E+14 | 2.42E-13 | 4.16E+01 |
| Am-241 | 7.65E+13 | 9.04E-13 | 6.91E+01 |
| Cm-242 | 9.93E+09 | 9.95E-13 | 9.88E-03 |
| Cm-244 | 1.21E+14 | 9.46E-13 | 1.14E+02 |
| Total | 1.53E+03 | ||
*Above values for 45GWd/t U burn up and 8-year cooling post reactor.
Table 1: Heat rating factor for some radioisotopes present in spent fuel dissolver liquor*.
The dissolver liquor would have a heat load of only 260W/t U when strontium isotopes and Y-90 daughter isotope are removed. Separation of cesium isotopes from dissolver liquor onto, for example, 1000 litres of adsorbent (~7.05 kW/1000l) would produce a highly active solid waste. After 120 years (~4 half-lives) of storage would be ~ 0.85 kW/1000l of adsorbent. This value is well below the HLW heat rating of < 2 kW/m3 [10]. The net result is that UCLan’s ART concept would not produce high-level waste if the separated cesium isotopes were stored for about 100 years prior to disposal, thus reducing the heat load on a geological disposal facility.
Selective cesium removal
The removal of cesium and/or strontium isotopes from spent fuel dissolver liquor has not been previously attempted. Various inorganic materials have been examined for removal of fission products, in particular cesium, from high active waste liquors but this liquor does not contain neither uranium nor plutonium isotopes [11,12]. UCLan’s process (ART) requires adsorbent materials that can accommodate much greater concentrations of uranium/plutonium i.e., have a very high cesium selectivity and in addition, the adsorbent requires high stability to nitric acid and radiation. Our previous publications have shown that commercial ion exchangers would not meet these criteria [13,14].
Ammonium Phosphomolybdate (AMP) is the only adsorbent/ion exchange material that can selectively remove cesium from high nitric acid concentration solutions and retain its stability and functionality. Smit et al. were the first to investigate the exchange of monovalent alkali metal ions by ammonium ions from AMP, the study concluded that AMP had excellent distribution coefficient values (kd) and good selectivity for cesium ions compared to other ions in up to 10 M HNO3 media [13- 15]. The challenge for UCLan is for the selective removal of cesium from spent fuel dissolver liquor, i.e., a liquor that contains significantly greater concentrations of uranium and plutonium (Table 2).
| Radionuclide | Approximate Concentration |
|---|---|
| U | ~300 g/L |
| Pu | 3.6 g/L |
| Np | 170 mg/L |
| Am | 225 mg/L |
| Cm | 8 mg/L |
| alkali metals (Cs, Rb) | 1.3 g/L |
| alkaline earth metals (Sr and Ba) | 1.05 g/L |
| Y and lanthanides | 4.4 g/L |
| Zr | 1.5 g/L |
| Se and Te | 220 mg/L |
| Mo | 1.4 g/L |
| Tc | 350 mg/L |
| Ru, Rh, Pd | 1.8 g/L |
| Ag, Cd, Sn. Sb | 60 mg/L |
*Concentrations are based on a typical irradiated PWR 3.7% U-235 fuel with a burn up of 45GWd/t HM, cooled for 3 years [19].
Table 2: Dissolver liquor concentrations*.
Although AMP as one of the major attributes for a cesium adsorbent i.e., high kd values, it is nonetheless a micro-crystalline material [16] thus limiting its practical applications; by incorporation of AMP with/within a polymeric matrix, that has resistance to strong acid and radiation degradation, these limitations may be overcome. Polyacrylonitrile (PAN) is such a material, Sebesta et al. first prepared AMP-PAN composite by using PAN as a binding or support material for AMP [17] which overcame its microcrystalline structure, producing a granular form that would be more suitable for column chromatography.
All chemicals were purchased from Sigma Aldrich and were of ANALAR grade.
Preparation of composite material
The AMP-PAN composites were prepared by same method as previously reported Park et al. [18]. The synthesis of AMP (50%) -PAN composite was achieved by dissolving 0.8 g of Tween 80 in 200 mL of dimethylsulfoxide (DMSO) and mixed at 50°C by overhead stirrer at approximately 250 rpm. 20 g of ammonium phosphomolybdate (AMP) was added to the solution and the mixture was stirred for a further 1 hour at 50°C. After one hour, a homogeneous yellowish green colour mixture was obtained, to which 20 g of polyacrylonitrile (PAN) powder was added and the solution was maintained at 50°C with constant stirring (~250 rpm) for 6 hours. The composite mixture was dispensed gravimetrically via a 1 mm (int. dia.) bore tube into ~400 mL of deionised water. The spheres were left overnight in deionised water and thereafter washed 3 times with fresh deionised water every 30 minutes. The washed beads were separated and dried in an air oven at 60°C for 24 hours
Cation uptake
As UCLan’s radioactive authorisation does not permit the handling of a ~300g/L uranium solution, or plutonium isotopes, cerium (IV) was used as a surrogate. Single ion solutions containing 5 mM of respective nitrate salt i.e., CsNO3, Ce(NO3)3 and (NH4)2Ce (NO3)6 were prepared, and used for the majority of the batch experiments (Table 3). In a 50 mL Duran glass bottle a 0.5 g of AMP (50%) PAN composite was contacted for 48 hours with 25 mL of the candidate solution in the Julabo SW ZZ water bath set at 25°C and 200 rpm. Samples of liquor were taken at discrete intervals up to 48 hours (assumed equilibrium had been attained) for rate of uptake experiments and analysed by ICPMS (Thermo; X series, Software; Plasma Lab.). Triplicate analytical data points were obtained for all ICP MS samples with average standard deviations from σ0.001 to 0.047. The experimental results in which cesium and cerium ions were present, used for capacity and selectivity measurements are reported in Table 3 and Table 4 below.
| Target cation | Nitric acid concentration (M) | ||
|---|---|---|---|
| 0.5 | 1 | 3 | |
| Cs kd | 135 | 132 | 101 |
| capacity (mg/g) | 25.7 | 25 | 26.8 |
| Ce kd | <0.2 | <0.2 | |
| Capacity (mg/g) | <0.1 | <0.1 | |
Table 3: Caesium and Cerium kd and capacity measurements.
| Ratio of Ce to Cs in solution | 20:1 | 50:1 | 450:1 |
| Cs kd value | 972 | 7748 | 4183 |
| Ce kd value | 1.38 | 5.32 | 1.09 |
| Composite selectivity for Cs:Ce | 704 | 1456 | 3830 |
Table 4: Selectivity of AMP (50%) PAN for Cs to Ce in 3.0 M nitric acid.
The experiments were designed to measure
The cesium capacity of the AMP PAN composite
Cesium rate of uptake
Cesium selectivity compared with cerium
Composite acid stability
The capacity, distribution coefficient and rate of uptake results were obtained by measuring the cation concentration of the solutions before and after equilibration. The concentration of Mo was also measured for some samples to determine the stability of the AMP (50%) PAN composite in nitric acid solutions.
The distribution coefficient (kd) was determined using
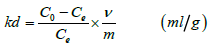 (1)
(1)
and the capacity (q)
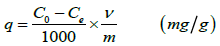 (2)
(2)
Where, Co = Initial concentration (ppm)
Ce = Final concentration (ppm)
v = Volume of the liquor (mL)
m = Mass of the sample (g)
For selectivity
SF = kd(Ce) / kd(Cs) (3)
AMP composite structure
The detailed x-ray crystal structure of AMP was first reported by Illingworth and Keggin [19]. According to their findings, the phosphomolybdate complex (PMo12O40)3- consists of a hollow sphere formed by 12MoO66- octahedra with PO43- group in the centre of the crystal structure of the ammonium salt of this ion (Figures 3a and 3b). The ammonium ions with associated water molecules probably fit in these spheres. Spheres (beads) of 1.5 to 2 mm (Figure 4a) were produced with a surface area of 15.05 m2/g and internal structure as shown in Figure 4b.
The AMP-PAN composite had a distinctive morphology; the outer surface was smooth, however, the bisection of spheres revealed continuous channel like structure, consistent with previously published information [20]. This structure could be due to the influence of surfactant on pore formation, the surfactant removed during the washing step. The large pores observed in the core of the AMP (50%)- PAN spheres, may be due to inclusion of air bubbles during the preparation of the spheres.
Cesium capacity
Cesium and cerium (surrogate for uranium/plutonium) uptakes were measured in 0.5, 1.0 and 3.0 M nitric acid solutions; cesium had good uptakes (kd values >100) whereas cerium had extremely low kd values. The kd values and equivalent capacities are reported in Table 3. The measured cesium kd values in this study were largely unaffected by acid concentration (Table 3) which is consistent with previously published work [21]. The maximum capacity for cesium on unsupported AMP varies from 1.6 to 0.8 mmoles/g most researchers have found the value to be ~0.95 mmoles/g [22] rather than the theoretical of 3 mmoles/g. Based on this previous published work then for AMP (50%) PAN a capacity of ~0.5 mmoles/g should be the maximum and hence the composite produced at UClan has achieved ~45%.
Cesium rate of uptake
Although acidity of the aqueous phase of 1.0 and 3.0 M as little effect, on the Cs uptake kinetics (Figure 5) the rate may be a little too slow for practical purposes and therefore needs improvement for commercial purposes. This comparative slow uptake of UCLan’s AMP (50%) PAN composite could be due to the internal structure of the sphere, which had a total pore volume of only 0.032 cm2/g. Higher internal porosity may provide some improvement in ion exchange performance.
Cesium selectivity
The selectivity of cesium over cerium is nearly 4000 at a ratio of 450 Ce:1 Cs, i.e., roughly the ratio of U to Cs in dissolver liquor (Table 4). This selectivity ratio should ensure that cesium would be preferentially extracted from spent fuel dissolver liquor without interference from uranium and plutonium [23]. One of the attributes to explain the separation factor for Cs and Ce could be their different metal ion size, hydrated Cs (12 coordination) is of the order 1.91 Å compared with Ce(iii) {tricapped trigonal prism} of 1.20 Å and Ce(iv) {square antiprism} of 1.07 Å [24]; the larger monovalent cation being preferred.
Acid stability
Previously published results confirmed that the properties of PAN binder and the AMP active component do not rapidly deteriorate in contact with strong acid solution and are equally robust at 106 Gy radiation dose [25]. The acid stability measurements under taken in this study also demonstrate a good degree of acid stability which is based on the concentration of Mo found in solution post contacting with cesium solutions (Table 5) for 24 hours. The % Mo loss value is based on the theoretical Mo content of AMP (61.3% Mo for (NH4)3 PMo12O40) The leaching of AMP from the AMP PAN matrix measured by the Mo loss is significantly lower than previous published data for encapsulated AMP [26] and far lower than the solubility of AMP alone in 1M nitric acid [27].
| Nitric acid concentration (M) | 0.5 | 1 | 3 |
| Mo solubility (ppm) | 2.6 | 3.0 | 6.7 |
| Loss from composite (%) | 0.021 | 0.024 | 0.055 |
Table 5: Acid stability values of AMP (50%) PAN.
Adsorption isotherm and kinetic model
The cesium uptake by AMP PAN composite is a function of both the temperature and concentration of cesium. Figure 6 show the experimental adsorption isotherm AMP PAN 50%. This isotherm indicates that for a typical spent fuel dissolver liquor (~800 mg/L cesium in 3 M nitric acid) the maximum capacities would be ~27 mg/g i.e., about 30 g to treat 1litre of dissolver liquor. The removal of cesium isotopes by AMP PAN would therefore require ~3.0 kg/t dissolved U.
Freundlich and Langmuir equations are often used to describe experimental isotherm data. The Freundlich isotherm model (equation 4) which involves the heterogeneity of sites and the exponential distribution of sites and their energies can be expressed as:
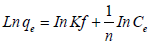 (4)
(4)
Where qe is the amount of cesium adsorbed by per gram of AMP PAN composite (mg/g)
Ce is the equilibrium concentration (mg/L)
Kf (mg/g) is the Freundlich constant and n is the Freundlich exponent.
Hence, a linear plot of ln qe versus ln Ce indicates that the adsorption follows the Freundlich isotherm model.
In comparison, the Langmuir sorption isotherm (equation 5) is often used to describe the sorption of a solute from a liquid solution and is valid for homogeneous and monolayer adsorption onto a surface with a finite number of identical site and can be represented by:
Ce/qe=Ce/(Q°)+ 1/bQ° (5)
A linear plot of Ce/qe versus Ce (Figure 7) shows that the adsorption obeys Langmuir isotherm model. Using data produced in these studies, Langmuir, Freundlich, Temkin and Elovich adsorption isotherms were produced for AMP PAN 50% but the highest correlation coefficient (R2) was obtained for the Langmuir model and the parameters calculated from the slopes and intercepts of this plot are reported in Table 6. The Langmuir constant Q° (mg/g) is consistent with experimental data.
| Langmuir constants | Q° (mg/g) | b (L/mg) | R2 |
|---|---|---|---|
| AMP PAN 50% | 27.1 | 0.462 | 1.0 |
Table 6: Parameters of Langmuir isotherm AMP PAN 50% composite at 25°C.
An understanding of the kinetics of adsorption is fundamental for selecting the optimum operating conditions as well as for designing and modelling of the process. The kinetics of the data reported in Figure 7 were analysed using different kinetic models; pseudo-firstorder, represented by;
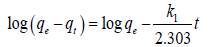 (6)
(6)
Where the term k1 (h-1) is the first order adsorption rate constant,
qe and qt the amount of cesium adsorbed at equilibrium and amount adsorbed at time t respectively.
For pseudo-second-order the rate is directly proportional to the number of active surface sites and rate expression can be written as:
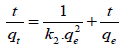 (7)
(7)
Where k2 (g/mg/h) is the rate constant, qe and qt are the amounts of cesium adsorbed at equilibrium and time t respectively.
Plots of;
(qe-qt) versus time at different acid conditions and t/qt against t are linear relationships.
Figure 7 profile data were used to construct first and second order kinetic models (equations 6 and 7 respectively), demonstrated that a pseudo second order model was preferred (R2 for 1st order 0.8224 against 0.9959 for 2nd order) (Figure 8).
These preliminary studies provide sufficient confidence that AMPPAN composite is an appropriate candidate for the selective removal of cesium isotopes from spent fuel dissolver liquor, thus reducing its heat load. Positioning UCLan’s chromatographic cesium separation process (ART) upstream of a PUREX process would greatly reduce;
1. Degradation of the TBP extractant
2. Solvent washing requirements
3. Number of downstream stages and shielding
4. GDF heat loads
Cesium uptake by the AMP PAN 50% composite was pseudo second order and obeyed the Langmuir adsorption model. The selectivity of the composite for cesium in comparison to cerium was in excess of 900 depending on aqueous phase conditions and was stable in 3 M nitric acid.
The authors are indebted to the University of Central Lancashire for its financial support of PK; to the EPSRC for funding AH (EP/ MO 26469/1), DR (EP/ LO18616/1) and to other members of the UCLan nuclear team for their assistance and contributions. The authors are grateful to Dr N Gribble (NNL) for provided heat decay data.