
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2024)Volume 14, Issue 5
Purpose: The purpose of the study is the presentation of the experience of one surgical team in patients with pancreatic cancer and peritoneal metastases treated with Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) and a review of the literature.
Methods: The data of patients with pancreatic cancer and peritoneal metastases who underwent treatment with CRS plus HIPEC were analyzed. Clinical and histopathologic variables were analyzed to predict survival, recurrence, and morbidity.
Results: In 10 patients (6 men and 4 women), with a mean age of 54.5 ± 12.2 (28-72) years, 13 cytoreductions and HIPEC were undertaken for pancreatic cancer and peritoneal carcinomatosis. Complications were recorded in 8 patients, and 2 patients died in the perioperative period. The 1 and 3 year overall survival rates were 76% and 18%, respectively, and the median survival was 28 months. The completeness of cytoreduction and the performance status were related to survival (p<0.05). The recurrence rate was 69.2%. The gender and the presence of ascites were related to recurrence (p<0.05). Ascites has been identified as a possible prognostic indicator of recurrence (p=0.027).
Conclusion: There is evidence that CRS with HIPEC may increase survival in selected patients with pancreatic cancer and peritoneal metastases. Future studies are needed to identify the group of patients that will benefit from this treatment.
Pancreatic cancer; Peritoneal metastases; Cytoreductive surgery, HIPEC
The peritoneum is the second most frequent site of recurrence from pancreatic cancer after the liver. Peritoneal metastases are present in 14% of the cases at initial diagnosis [1], and in 50% at the time of death [2,3]. Improvements in systemic chemotherapy have increased the overall survival of patients with peritoneally disseminated pancreatic cancer. The overall survival still remains low and does not exceed 9 months [4]. Peritoneal dissemination is classified as stage IV disease, and only systemic palliative chemotherapy is considered the standard treatment [5].
CRS with HIPEC has been shown to be an effective treatment for many diseases with peritoneal dissemination [6-10]. CRS is performed with the intent of resecting the entire macroscopically visible tumor, and HIPEC is integrated in CRS with the intent to eradicate the microscopic residual tumor, which almost always remains at the peritoneal surfaces even after complete cytoreduction. The use of HIPEC has been shown to be feasible and safe after pancreatic resection [11,12].
The extent of peritoneal disease and the completeness of cytoreduction have been identified as the most significant variables of survival for diseases with peritoneal malignancy [7-10]. The most recent publication with pancreatic cancer and peritoneal metastases has shown encouraging results in properly selected patients with a limited extent of peritoneal disease who have undergone complete cytoreduction [13]. The presence of metastatic liver disease in pancreatic cancer is no longer considered an unresectable disease ex-principio [14]. A sub-group of pancreatic cancer patients with liver oligo-metastatic disease may be offered survival benefit from surgery. The limit of surgery in these situations is still unknown.
The purpose of the present study is to update the results of a surgical team with a limited experience in the treatment of pancreatic cancer with peritoneal metastases and to review the literature.
Patient methods
The data of the patients with peritoneal malignancy from pancreatic cancer was retrospectively reviewed in a prospectively maintained database. The patients were treated in an accredited Department of Surgical Oncology specialized in Peritoneal Surface Malignancy by the same surgical and anesthesiological team. All patients signed an informed consent indicating that the treatment was not in routine practice, and did not provide established benefit. The ethical committee of the hospital approved the protocol as well as the publication of the manuscript.
Preoperative work-up included physical examination, hematologic- biochemical examinations, tumor markers (CEA, CA 19-9, CA- 125), abdominal and thoracic CT scanning with the intent to identify the presence of unresectable metastatic disease, the precise site of implants, and calculate the extent of the peritoneal disease. The performance status was assessed according to the Karnofsky performance scale. The anesthesiological assessment classified the patients according to ASA stage. Diagnostic laparoscopy and/or CT-enteroclysis were also used in those cases in which the extent of the peritoneal disease at the small bowel was inconclusive. The presence and the volume of ascites were recorded in detail in every case, as well as the location of the primary tumor. The extent of prior surgery was assessed according to previous official surgical report using the Prior Surgery Score (PSS). PSS-0 defined those patients who had not undergone surgery previously. PSS-1 defined those patients who had undergone biopsy or surgery in one abdominopelvic region, PSS-2 those who had undergone surgery in 2-5 abdominopelvic regions, and PSS-3 those with surgery in >5 regions [15]. The tumor volume was also assessed. LS-0 defined no visible implants on a specific abdominopelvic region. LS-1 defined the presence of implants with their largest diameter <0.5 cm. LS-2 defined the presence of implants with the largest diameter >0.5 cm and <5 cm, while LS-3 defined implants with their largest diameter >5 cm or confluent implants of any size. Patients with implants LS-1 were considered as having small volume tumors and those with implants LS-2 and LS-3 as having large volume tumors.
Eligibility criteria
Patients over 16 years, with acceptable performance status (Karnofsky scale>50%), ASA-stage <III, with normal renal function (blood urea <50 mg/dl, and creatinine <1.5 mg/dl), white blood cell count >4000, platelets >100,000, normal hepatic function, and capable of undergoing major surgery were considered eligible for treatment. Pregnant women, or patients with a recent history of cardio-pulmonary disease, poor performance status (Karnofsky scale <50%), ASA-stage >III, or with an abnormal renal-hepatic- hematologic profile were excluded from treatment. Addictive or psychotic patients were also excluded. Patients who could undergo complete cytoreduction were considered candidates for surgery. Extensive seeding of the mesentery of the small bowel, involvement of the antimesenteric edge of the small bowel, or tumor >5 cm at its largest diameter at the Treitz ligament were exclusion criteria.
Surgery
A midline incision extending from the xiphoid process to the symphysis pubis was used for maximal exposure of the abdominal cavity. The extent of the peritoneal disease was calculated using the Peritoneal Cancer Index (PCI) after lysis of the adhesions. Cytoreductive surgery was possible using the standard peritonectomy procedures [16]. The completeness of cytoreduction was calculated after tumor resection according to Sugarbaker’s criteria [15]. Radio- Frequency Ablation (RFA) was used for the eradication of liver metastatic lesions. HIPEC was administered with the Coliseum technique (open abdominal technique) with a continuous closed circuit of four drains (two inlet and two outlet), one heat exchanger, and two roller pumps connected to the inlet and outlet drains (Sun-Chip, Gamida Tech, Paris, France). The cytostatic drugs were delivered diluted in 2-3 liters of Normal Saline or Ringer’s Lactate at 42.5 C-43.0 C. HIPEC with gemcitabine was performed for 60 min and with Mit-C for 90 min. One patient received intravenously 5-FU (400 mg/m2) plus Leucovorin (20 mg/m2) concurrently to perfusion. The reconstruction of the continuity of the alimentary tract was always performed after the completion of HIPEC.
All patients remained in the ICU for at least 24 hours after surgery. Postoperative complications were carefully recorded and classified according to the Clavien-Dindo classification [17].
All specimens were histopathologically examined in detail. The histologic subtype was defined. The number of the resected and infiltrated lymph nodes was also recorded, as well as the infiltration of nerves and veins.
Follow-up
The patients were followed every 4 months for the first year and every 6 months later until death with physical examination, hematological-biochemical examinations, tumor markers (CEA, CA 19-9, CA-125), and radiologic examinations (thoracic and abdominal CT-scan). The time and the site of recurrence were recorded.
Statistics
Statistical analysis was performed using the SPSS (Statistical Package for Social Sciences, version 17.0). The proportions of patients with a given characteristic were compared by x2 or by Pearson’s test. The Kaplan-Meier method was used for the construction of survival curves. The comparison of curves was possible using the log-rank test. Multivariate analysis of survival was assessed with the Cox proportional hazard model for the identification of the independent variables of survival. Logistic regression analysis was used to identify the independent variables of recurrence and morbidity. A two-tailed p value <0.05 was considered statistically significant.
The files of 10 patients (6 men and 4 women) with pancreatic cancer and peritoneal metastases who underwent 13 cytoreductions from 2011-2018 were retrieved. The mean age of the patients was 54.5 ± 12.2 (28-72) years. Three patients underwent secondary cytoreduction additionally because of recurrence.
The general characteristics of the patients are listed in Table 1. The primary tumor was located in the pancreatic tail in almost all cases. The mean PCI was 10 ± 5 (3-20). All patients had a large volume tumor, and ascites was present in 5 of them. Three patients were identified with peritoneal metastases at the initial diagnosis, and one of them had 6 synchronous hepatic metastatic lesions, while the others were found with metachronous peritoneal metastases. Two patients with a large volume tumor in the small bowel were not considered candidates for CRS at the time of initial diagnosis and received neo-adjuvant chemotherapy. One of them was given gemcitabine and abraxane and the other FOLFIRINOX. Both patients responded after 4 cycles of chemotherapy and were considered eligible for CRS plus HIPEC. In addition, two women had been previously treated with CRS and systemic chemotherapy for ovarian cancer and presented with peritoneal carcinomatosis in 2-3 years after initial treatment. The radiologic examinations were inconclusive in regard to the origin of the peritoneal disease. The tail of the pancreas was enlarged without any obvious tumor in both patients. Complete cytoreduction (CC-0) was possible in 11 cases. Epigastric peritonectomy procedure (resection of the previous scar with the round and the falciform ligaments of the liver) was undertaken in 1 case. Right and left subdiaphragmatic peritonectomies were undertaken in 5 and 3 cases, respectively, greater and lesser omentectomy in 7 and 5 cases respectively, and splenectomy in 5 cases. Cholecystectomy and resection of the omental bursa was undertaken in 4 cases. Right and left lateral peritonectomies were performed in 5 and 4 cases, respectively, while pelvic peritonectomy was necessary in 6 cases. In addition, subtotal gastrectomy was undertaken in 3 cases, subtotal colectomy in 2, segmental intestinal resection in 3, right colectomy in 1, hepatic RFA in one case, and in another one, resection of the left kidney was required in order to achieve CC-0 surgery. Distal pancreatectomy was undertaken in 5 cases. In 2 cases, a distal pancreatectomy had been previously performed. Additional pancreatectomy was performed during reoperation. One patient was treated with palliative surgery (CC-3). The patient presented with a complete obstruction of the small bowel and underwent a by-pass procedure because the small bowel was extensively seeded, although a CC-0 surgery had been previously achieved. This patient did not receive HIPEC. During perfusion, gemcitabine (1000 mg/m2) was given in 7 cases, and a combination of cisplatin (50 mg/m2)+Mit-C (10 mg/m2) in 3 cases. Postoperative systemic chemotherapy with gemcitabine was administered in 8 cases. The diagnosis of pancreatic ductal adenocarcinoma was established in 11 specimens by histopathology. Peri-pancreatic lymph nodes were positive in all specimens as well as neural and venous infiltration. One patient was diagnosed with pancreatic adeno-squamous tumor and underwent CRS and HIPEC twice because of recurrence.
| No of pts | % | |
|---|---|---|
| Tumor volume | ||
| Large volume | 13 | 100 |
| Small volume | 0 | 0 |
| Ascites | 5 | 38.5 |
| PSS | ||
| PSS-0 | 5 | 38.5 |
| PSS-1 | 0 | 0 |
| PSS-2 | 5 | 38.5 |
| PSS-3 | 3 | 3 |
| PCI | ||
| <13 | 9 | 69.2 |
| >13 | 4 | 30.8 |
| CC | ||
| CC-0 | 11 | 84.6 |
| CC-1 | 1 | 7.7 |
| CC-2 | 0 | 0 |
| CC-3 | 1 | 7.7 |
| Morbidity | 8 | 61.5 |
| Mortality | 2 | 15.4 |
| Recurrence | 9 | 69.2 |
| Site of recurrence | ||
| Distant | 5 | 38.5 |
| Local-regional | 4 | 30.8 |
Table 1: General characteristics of patients.
Complications were recorded in 8 cases (61.5%) (Table 1). Two patients had Grade I complications, one patient had Grade II complication, 3 had Grade III, and 2 had Grade V complications. One patient, who was a heavy smoker, required prolonged mechanical ventilation due to pulmonary insufficiency. The same patient developed recurrence in one year, and despite treatment with systemic chemotherapy, he underwent secondary CRS and HIPEC. He died on the 5th postoperative day because of acute hepatic failure. One patient who underwent CRS and HIPEC twice presented severe bile esophagitis as a result of proximal gastrectomy and esophagogastric anastomosis. Another patient was complicated by an enterocutaneous fistula, and two more patients developed intra-abdominal abscesses because of pancreatic leaks (one of them presented with a delayed abscess 4 months after surgery). One more patient died on the 6th postoperative day due to renal failure as a result of intraoperative hemodynamic instability.
Survival
The median survival was 28 months. The 1 and 3 year overall survival rates were 76% and 18% respectively (Figure 1). Univariate analysis of survival showed that the completeness of cytoreduction and the performance status were correlated to survival (Table 2). Multivariate analysis did not identify any possible independent variable of survival. No variable was found to be related to morbidity (Table 3). The median disease-free survival was 23 months.
| Survival | Morbidity | |
|---|---|---|
| Variable | P value | P value |
| Gender | 0.93 | 0.569 |
| PSS | 0.143 | 0.42 |
| PCI | 0.475 | 0.569 |
| CC | 0.012 | 0.151 |
| Performance status | 0.018 | 0.42 |
| ASA stage | 0.561 | 0.057 |
| Age | 0.838 | 0.224 |
| Ascites | 0.523 | 0.279 |
| Morbidity | 0.109 |
Table 2: Univariate analysis of survival.
| Univariate analysis | Multivariate analysis | |
|---|---|---|
| Variable | P value | P value |
| Gender | 0.039 | |
| PSS | 0.231 | |
| PCI | 0.338 | |
| CC | 0.762 | |
| Morbidity | 0.164 | |
| Performance status | 0.415 | |
| ASA stage | 0.425 | |
| Age | 0.197 | |
| Ascites | 0.039 | 0.027 |
Table 3: Analysis of recurrence.
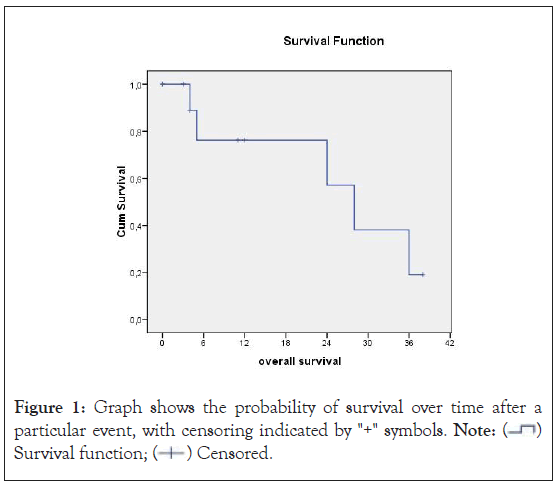
Figure 1: Graph shows the probability of survival over time after a particular event, with censoring indicated by "+" symbols. Note:  Survival function;
Survival function;  Censored.
Censored.
Follow-up
The median follow-up time was 11 months (3-38). Recurrence was recorded in 9 cases (69.2%). There were 5 distant (38.5%) and 4 local-regional recurrences (30.8%). The gender (p=0.039) and the presence of ascites (p=0.039) were found to be related to recurrence by univariate analysis. The presence of ascites (p=0.027) has been identified as a possible prognostic variable of recurrence by multivariate analysis.
Currently, 1 patient (9.1%) remains alive without disease 38 months after initial treatment, 5 (45.5%) died because of disease recurrence, 1 patient (9.1%) died because of reasons unrelated to the disease, and 4 patients (36.4%) are alive with disease recurrence.
Despite improvements in the outcomes of pancreatic cancer surgery, the overall survival has not significantly increased. The results of the administration of chemotherapy (adjuvant or neo-adjuvant), or immunochemoradiotherapy are controversial [18]. Up to 70% of patients with surgical resection develop local-regional recurrence in 2-3 years [19]. The 5-year survival after R0 resection combined with multimodality treatment does not exceed 20% in high-volume and specialized centers [20].
Until recently, patients with metastatic pancreatic cancer were excluded from surgery [18]. The synchronous resection of both the primary and the peritoneal metastatic tumors appears to offer a significant survival benefit [13,14]. The untreated peritoneal metastases of pancreatic cancer origin usually lead very soon to intestinal obstruction, ascites, and malnutrition [21].
The pathophysiology of peritoneal metastases remains unclear. After disruption of the pancreatic serosa, cancer emboli exfoliated from the surface of the primary tumor move to remote sites assisted by the peritoneal fluid motion, the intestinal motility, the respiratory movements, and the gravity. Some of them are implanted at the peritoneal surfaces, and others are absorbed by the greater and lesser omentum or the hemidiaphragms and progress to visible peritoneal metastases. Low-volume local disease progresses to visible peritoneal implants in patients who have been left with positive margins of resection. On the other hand, cancer emboli originate from the traumatized interstitial tissues located within narrow limits of resection, or from the transected lymphatic network, or even from venous blood lost during surgical manipulations. During wound healing, the emboli entrapped in fibrin attract inflammatory cells and collagen, and stimulated by growth factors give rise to recurrent tumors in 2-3 years after initial surgery [22]. Systemic chemotherapy is ineffective in the control of local-regional recurrence [23]. Experimental work has shown that the intraperitoneal administration of gemcitabine may effectively control the local-regional microscopic tumor [24]. A high drug level with low systemic exposure is achieved by the intraperitoneal administration of cytostatic drugs [25], while the high concentration of the absorbed drug in the portal circulation may possibly eradicate the micrometastatic hepatic disease [26]. There is evidence from previous clinical studies that local- regional control of the microscopic residual pancreatic tumor may be possible with intraperitoneal chemotherapy [27-29]. The spontaneous development of peritoneal metastases in patients with tumors of the pancreatic tail has not been thoroughly explained. Peritoneal metastases in the lesser peritoneal sac may reasonably be found. Inexplicably, even in patients without a history of previous abdominal operation, peritoneal metastases are identified in the greater peritoneal sac and not necessarily in the lesser peritoneal sac.
Farma et al. presented discouraging results in an older study in which a small number of patients with gastric, duodenal, and pancreatic cancer were included together [30]. The beneficial effect of CRS and HIPEC has been shown in a previous report of case series. In this study, there was no limit in regard to the extent of the peritoneal disease, and long-term survival was reported even for patients with extensive disease who did not undergo complete cytoreduction. All tumors were located in the tail of the pancreas [31]. In a recent comparative study, Gudmundsdottir et al. showed that patients with limited peritoneal extent undergoing complete cytoreduction and HIPEC have 1-, 2-, and 3-year survival rates of 91%, 66%, and 59% respectively, with the longest survivor at 54 months without evidence of disease. The results of the study are outstanding, showing that pancreatic cancer with peritoneal metastases is not always a lethal disease, and possibly there is a glimpse of hope for long-term survival for a subgroup of patients. In this study, the group of CRS plus HIPEC consisted of 5 patients with tumors of the head of the pancreas and 18 with tumors of the tail [13]. These results are in contrast to Artinyan et al., who have shown that patients with tumors of the body and tail of the pancreas have a worse prognosis than those with tumors of the head and are associated more frequently with hepatic metastases [32]. Gudmundsdottir, et al. strongly support the routine use of preoperative laparoscopy in every patient with pancreatic cancer with the intent to identify small volume peritoneal metastases which are otherwise undetectable by radiologic examinations. These patients, as well as those with positive peritoneal cytology without visible peritoneal metastases, may undergo complete CRS and HIPEC. Another option for detecting small peritoneal metastases undetectable with the conventional imaging is the use of CT enteroclysis, which has 92% sensitivity, 96% specificity, 97% positive predictive value, and 91% negative predictive value in the assessment of peritoneal metastases at the small bowel and its mesentery [33]. There is much evidence that CRS and HIPEC are effective in the treatment of pancreatic cancer with peritoneal metastases. However, the cut-off point of the PCI has not yet been identified. The completeness of cytoreduction score is the most significant prognostic variable for long-term survival, regardless of the primary tumor origin [6-10]. In our limited experience one patient who underwent incomplete (CC-1) cytoreduction survived more than 30 months [31]. In our updated study, we included one patient with large volume peritoneal disease and hepatic metastatic lesions who underwent complete cytoreduction plus ablation of the hepatic disease and survived 28 months. There is evidence that pancreatic cancer resection may be performed concurrently with liver metastatic disease [14]. The resection of liver metastatic disease and colorectal cancer at the same time has been shown to be feasible and beneficial [34]. Future studies are needed to answer if patients with resectable pancreatic cancer and synchronous peritoneal and liver metastases may be offered a survival benefit by undergoing concurrent resection or ablation to CRS and HIPEC.
The rate of morbidity was high. Univariate analysis did not reveal any variables related to morbidity. The ASA stage is probably an exception because it showed a trend to correlate to morbidity (p=0.057). In a recent review study, Brind’Amour et al. have reported that CRS and HIPEC appear to be a safe method, conferring the same rate of morbidity and mortality as surgery for pancreatic cancer without peritoneal metastases. The method appears to offer local control in highly selected patients [35]. No hematologic toxicity was recorded, in contrast to Satoi et al., who reported 42% hematologic toxicity Grade III/IV and 18% non- hematologic adverse effects [36]. Nevertheless, despite the use of systemic chemotherapy, the rate of recurrence was high (69.2%) and distant metastases slightly prevailed.
This is a study that does not intend to draw definitive conclusions about the use of CRS and HIPEC in pancreatic cancer patients with peritoneal metastases. The medical literature and the present study provide evidence suggesting that CRS and HIPEC may be safely used. It remains unclear which is the subgroup of patients that may be offered a significant survival benefit. Hopefully, this will be the challenge for the future.
Funding
The authors declare that no funds, grands, or other support were received during the manuscript “Resectable Pancreatic Cancer with Peritoneal Metastases”
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Tentes AA, Kyziridis D, Kalakonas A, Courcoutsakis N (2024) Resectable Pancreatic Cancer with Peritoneal Metastases. J Clin Trials. 14:571.
Received: 20-May-2024, Manuscript No. JCTR-24-31561; Editor assigned: 22-May-2024, Pre QC No. JCTR-24-31561(PQ); Reviewed: 05-Jun-2024, QC No. JCTR-24-31561; Revised: 12-Jun-2024, Manuscript No. JCTR-24-31561(R); Published: 19-Jun-2024 , DOI: 10.35248/2167-0870.24.14.571
Copyright: © 2024 Tentes AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.