
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2024)Volume 14, Issue 2
Aim: Up to 20% of patients with COVID-19 get critically ill and require Intensive Care Unit (ICU) admission. At hospital discharge, most patients still have physical and mental limitations, which affect their quality of life. Pulmonary functional alterations in patients with COVID-19 vary from the absence of functional abnormalities to restrictive and diffusion impairments. We aimed to describe pulmonary function abnormalities as well as their impact on the 6-Minute Walk Test (6 MWT) and SF-36 Physical Component Summary (PCS) score in patients with COVID-19 at ≥ 3 months after hospital discharge.
Methods: We included 65 patients aged ≥ 18 years with severe COVID-19 confirmed through real-time reverse transcriptase-polymerase chain reaction and admitted to the ICU between April 2020 and October 2021. Patients were evaluated at ≥ 3 months after hospital discharge using the 6 MWT, Pulmonary Function Tests (PFTs), and the PCS score.
Results: Among the included patients, 27 patients had abnormal PFT findings, 21 (32.3%) had forced vital capacity<80%, 17 (26.1%) had forced expiratory volume in 1 s<80%, and 4 (6.1%) had a maximal mid-expiratory flow<65%. Compared with patients without abnormal PFT findings, patients with abnormal PFT findings were older and had significantly higher ferritin levels. There were no significant between-group differences in invasive and noninvasive respiratory support, mechanical ventilation duration, vasopressor use, and renal replacement therapy. However, compared with patients with normal PFT findings, patients with abnormal PFT findings showed a significantly lower 6-MWT score [78% (0.0–92) vs. 95% (75–100), p=0.01] and worse PCS scores [39.4 (32.1–51.3) vs. 52.0 (47.4–57.3), p=0.007]. There was an independent association between the PCS scores and PFT findings.
Conclusion: We found that a significant proportion of patients present pulmonary functional alterations ≥ 3 months after discharge from the hospital after treatment for severe COVID-19; further, these alterations affect physical functional capacity and quality of life.
SARS-CoV-2; Post-COVID; Pulmonary function test; Follow-up; Physical component summary; 6-minute walk test
ICU: Intensive Care Unit; 6-MWT: 6-Minute Walk Test; PCS: Physical Component Summary; SF- 36: Short form Survey for Quality of Life; PFT: Pulmonary Function Test; SAPS 3: Simplified Acute Physiology Score version 3; PaO2/FIO2: Ratio of arterial oxygen partial pressure (PaO2) to fractional inspired oxygen; KDIGO: Kidney Disease Improving Global Outcomes score criteria; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in the first second; MMEF: Maximal Mid-Expiratory Flow; DLCO: Lung Diffusion Capacity for Carbon Monoxide
The coronavirus 2019 (COVID-19) pandemic has affected>600 million people worldwide, resulting in ≥ 6 million deaths. Although its acute phase has been clinically characterized [1,2], the clinical evolution of patients with COVID-19 after hospital discharge remains unclear. Specifically, there are limited available data from follow-up studies. Preliminary studies have indicated that a substantial proportion of patients present with symptoms persisting for several months [3,4]. Some patients present with organ damage, especially in the lungs [5,6]. Recent studies have described the frequency and severity of pulmonary structural and functional changes after discharge in hospitalized patients with COVID-19 who received invasive and non-invasive respiratory support. These studies have demonstrated that a considerable proportion of these patients present constitutional manifestations [7,8], and tomographic alterations [7,9]. However, Pulmonary Function Tests (PFTs) in the patients either reveal normal findings or restrictive alterations or gas exchange alterations indicated by reduced Lung Diffusion Capacity for Carbon Monoxide (DLCO). Regarding physical performance, studies have reported changes in the 6-Minute Walk Test (6 MWT) scores [9]. There has been increasing interest in the clinical manifestations of COVID-19 at least 3 months after disease onset, which is termed as post- COVID-19 syndrome [10], given the high proportion of patients who remain with permanent sequelae. Since the lung is the main target organ of the disease in the acute phase, elucidating medium- to long-term pulmonary functional changes and their effects on the physical quality of life could inform patient rehabilitation programs [11,12].
Accordingly, we aimed to determine the prevalence of restrictive, obstructive, and small airway alterations in survivors of severe COVID-19 at least three months after hospital discharge. Further, we aimed to examine the impact of their spirometry patterns on the 6-MWT and Physical Component Summary (PCS) of the SF-36 quality-of-life instrument.
This retrospective observational cohort study included 65 patients aged ≥ 18 years with severe COVID-19 confirmed using real-time reverse transcriptase-polymerase chain reaction and admitted to a 35-bed intensive care unit of a tertiary hospital between April 1, 2020, and October 31, 2021. We excluded pregnant or breastfeeding women, patients receiving palliative care, patients with chronic obstructive pulmonary disease, and patients with symptomatic asthma. Upon hospital discharge, patients were invited for evaluation in our multidisciplinary follow-up clinic after 3 months. Here, the patients were evaluated using the 6-MWT, PFTs, and the PCS score for quality of life. The trial protocol was approved by the Research Ethics Committee of the Hospital Sao Domingos (Number 5.403.663) and registered in clinical trials. Gov (NCT05249842).
Data were obtained from the electronic medical records of the hospital. The demographic and clinical characteristics included age, sex, Simplified Acute Physiology Score 3 score, and comorbidities. The clinical and laboratory data upon ICU arrival included PaO2/FIO2, blood count, C-reactive protein, D-dimer, ferritin, fibrinogen, and lactic dehydrogenase. The drug interventions included corticosteroids, heparin, and tocilizumab. Further, we collected data regarding the characteristics of invasive and non-invasive respiratory support, use of the prone position, and vasoactive drugs. Additionally, we collected data regarding the main complications, acute kidney injury requiring hemodialysis, secondary infectious complications, and clinical outcomes. Acute renal injury was characterized using the Kidney Disease Improving Global Outcomes score criteria [13]. The presence of infectious bacterial or fungal complications was determined by the attending physician based on the laboratory (mainly procalcitonin), microbiological, and imaging data. The PFTs included Forced Vital Capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC ratio, and Maximal Mid-Expiratory Flow (MMEF) using a spirometer Spirobank II (MIR, Italy). The standardization and categorization of functional changes were based on Brazilian guidelines for PFTs [14].
Statistical analysis
Categorical variables are reported as frequencies and proportions. Normally and non-normally distributed quantitative variables are presented as mean (standard deviation) and median (interquartile range), respectively. We performed between-group comparisons of categorical variables using the χ2 test or Fisher’s exact test, as appropriate. For quantitative variables, the non-parametric Mann- Whitney U test or Student’s t-test was used.
A multiple logistic regression model was used to identify clinical characteristics related to pulmonary functional changes. Variables with p<0.20 in the unadjusted analysis were included in the model. Additionally, variables with p<0.05 in the adjusted analysis were considered significant.
Statistical significance was set at P<0.05. Statistical analyses were performed using the R software version 4.0.2 (R Core Team, 2017).
From April 1, 2020, to October 31, 2021, 689 patients were admitted to the ICU for COVID-19 at the Hospital São Domingos. Moreover, 358 and 332 patients were discharged from the ICU and hospital, respectively. Among them, 65 discharged patients were evaluated at the hospital’s ICU follow-up clinic within a median post-discharge period of 146 (120–183) days (Figure 1). The median age of the enrolled participants was 50 years (43–60 years), with 36 (55.3%) men and 29 (44.6%) women.
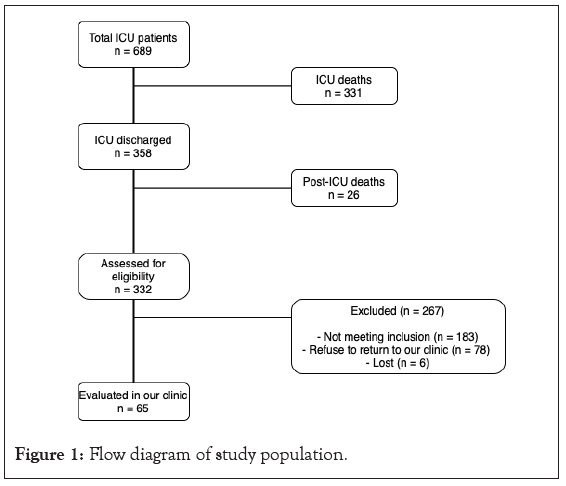
Figure 1: Flow diagram of study population.
The most common comorbidity was hypertension (22 patients, 33.8%), followed by diabetes (13 patients, 20%) and obesity (9 patients, 13.8%). In addition, 45 (69.2%) patients underwent invasive mechanical ventilation. Furthermore, 27 (41.5%) patients had abnormal PFT findings as follows: FVC<80% in 21 (32.3%) patients, FEV1<80% in 17 (26.1%) patients, FEV1/FVC<0.7 in 0 patients, and MMEF<65% in 4 (6.1%) patients. Table 1, shows the comparisons of the demographics, clinical severity, laboratory data, therapeutic interventions, and outcomes between patients with normal and abnormal PFT findings (n=38 and n=27, respectively). Compared with patients without abnormal PFT findings, patients with abnormal PFT findings were older and had significantly higher ferritin levels [1358 (635–1807) vs. 2000 (886–3000), p=0.02] (Table 1).
| Normal RFT N=38 | Abnormal RFT N=27 | p | |
|---|---|---|---|
| Age, y Median (IQR) | 54 (41.5-63.0) | 48 (44-54) | 0.04 |
| Males, n (%) | 21 (55.2) | 15 (55.5) | 0.23 |
| SAPS 3 score Median (IQR) | 51 (45-62) | 50 (46-60) | 0.35 |
| Comorbidities (n) | |||
| Hypertension | 15 | 7 | 0.13 |
| Diabetes | 7 | 6 | |
| Obesity | 4 | 5 | |
| Laboratory data at admission, Median (IQR) | |||
| D-dimer, mg/L | 1410 (710-8355) | 3240 (1200-8470) | 0.11 |
| Ferritin, ng/ml | 1358 (635-1807) | 2000 (886-3000) | 0.02 |
| Fibrinogen, mg/dL | 612 (521-734) | 662 (499-799) | 0.33 |
| PaO2/FIO2 | 172 (130-222) | 195 (129-256) | 0.47 |
| Life-sustaining support during ICU stay | |||
| Nasal O2/Venturi | 5 | 2 | |
| Non invasive ventilation | 9 | 4 | 0.65 |
| Invasive ventilation | 24 | 21 | 0.76 |
| Duration of IMV, d Median (IQR) | 12 | 10 | 0.67 |
| Vasopressors, n (%) | 19 | 18 | 0.61 |
| RRT, n (%) | 4 | 7 | 0.22 |
| Corticosteroids, n (%) | 24 | 22 | 0.86 |
| ICU LOS, d Median (IQR) | 13 (7-13) | 5 (2-7) | 0.07 |
| Hospital LOS, d Median (IQR) | 25 (18-39) | 34 (17-68) | 0.1 |
| Pulmonary function tests | |||
| FVC<80%, n (%) | 21 (32.3%) | ||
| FEV1<80%, n (%) | 17 (26.1%) | ||
| FEV1/FVC<0.7, (n %) | 0 (0%) | ||
| MMEF<65%, n (%) | 4 (6.1%) | ||
| Length of time to follow-up | |||
| ICU discharge/follow up, Evaluation, d Median (IQR) | 158 (140-187) | 146 (118-195) | 0.08 |
| Hospital Discharge/Follow-up Evaluation, d Median (IQR) 6-MWT (%) |
155 (134-176) | 138 (93-185) | 0.04 |
| 95 (75-100) | 78 (0.0-92) | 0.01 | |
| PCS score Median (IQR) | 52.0 (47.4-57.3) | 39.4 (32.1-51.3) | 0.007 |
Note: SAPS 3 Simplified Acute Physiology Score, PFT pulmonary function tests, 6-MWT 6-minute walk test, PCS physical component summary.
Table 1: Clinical characteristics and outcomes in patients with normal vs. abnormal Renal Function Tests (RFT) following ICU admission.
There were no significant between-group differences in invasive and noninvasive respiratory support, mechanical ventilation duration, vasopressor use, and renal replacement therapy. However, compared with the group with normal PFT findings, the group with abnormal PFT findings showed a significantly lower 6-MWT score [78% (0.0–92) vs. 95% (75–100), p=0.01] and worse PCS scores [39.4 (32.1–51.3) vs. 52.0 (47.4–57.3), p=0.007].
In the logistic regression model of PFT after 4 months with adjustment for independent covariates, multivariate analysis showed statistical significance for the PCS (p=0.01) and a tendency toward significance for the 6-MWT (p=0.09).
In our study, 41.5% of the patients presented abnormal PFT findings within a median post-discharge period of 4.8 months; additionally, these alterations affected the quality of life as indicated by the 6 MWT and PCS scores. Multivariate analysis revealed an independent association between lower PCS scores and abnormal PFT findings.
Several studies have examined lung functional changes in hospitalized patients with COVID-19 of varying severities. In a Spanish multicenter study, Blanco et al. analyzed 100 patients with mild/moderate or severe COVID-19 [15]; however, they excluded patients undergoing mechanical ventilation. The patients were evaluated within a median duration of 3 months after the onset of symptoms, with the PFTs only revealing changes in the DLCO. Fortini et al. analyzed 59 patients who were hospitalized in non-intensive wards after a median post-discharge period of 4 months [8]. They observed abnormal PFT findings, with the most common being a reduction in the DLCO (37% of the patients). Although they described the persistence of symptoms, they did not objectively analyze aspects regarding the quality of life at 4 months after discharge. Polese et al. analyzed changes in PFT findings in 41 patients hospitalized with severe COVID-19 within 36 days after the onset of symptoms [7]. They observed changes in the FVC and DLCO in>50% and 79% of the patients, respectively. Further, 45% of the patients showed a significant reduction in the distance covered in the 6 MWT. van Gessel et al. evaluated 48 hospitalized patients with severe COVID-19 who underwent invasive mechanical ventilation 3 months after hospital discharge [9]. They observed significant reductions in the total lung capacity and DLCO. Moreover, there was a high proportion of patients with pulmonary fibrosis on the computed tomography scan and a reduction in the distance covered in the 6 MWT. A meta-analysis of respiratory function after COVID-19 infection included seven studies and 380 patients [16]. Among them, 2,2,2 and 1 study performed follow-up assessments at 2 weeks after hospital discharge, 30 days after symptom onset, 1 month after hospital discharge, and 3 months after hospital discharge, respectively. DLCO, restrictive, and obstructive changes were identified in 39%, 15%, and 7%, respectively. Early evaluation may impede the accuracy of functional diagnosis since we cannot determine the extent of post-infection sequelae and acute inflammation. The British Thoracic Society recommends evaluation using PFTs ≥ 3 months after discharge [17].
The present study assessed the prevalence of small airway changes using the MMEF test, with four patients (6.1%) having values<65%. There have been inconsistent reports regarding small airway changes in patients with COVID-19. Lindahl et al. analyzed 20 patients with severe COVID-19 at 3 and 6 months after hospital discharge through impulse oscillometry and observed no small airway changes [18]. Cho et al. analyzed 100 patients with varying degrees of COVID-19 severity and found that 52% of patients showed tomographic changes suggestive of small airway involvement [19]. Future multicenter prospective studies are warranted.
Although the clinical manifestations of the acute COVID-19 phase and each evolutionary phase of the disease are well established, its long-term effects remain unclear. Recent studies have demonstrated that some patients with COVID-19 present with clinical manifestations that last for up to 4–12 weeks (symptomatic COVID-19) and>12 weeks (post-COVID-19 syndrome) after the onset of symptoms. In our study, we observed changes in the PFT, 6 MWT, and PCS scores, which can be described as post- COVID syndrome, in 41.5% of patients. Huang et al. analyzed 1733 patients at 6 months after symptom onset and found that 76% of patients had at least one symptom, with the most common being muscle weakness; however, others presented with anxiety, depression, cognitive changes, and reduced 6 MWT scores. Regarding pulmonary functional alterations, the most common alterations were observed in the carbon monoxide diffusion test; however, there were abnormal PFT findings that characterized a restrictive disorder.
Limitations
This study has several limitations. First, this was a retrospective single-center study, which accordingly involved limited volume and quality of information. Second, we did not evaluate the DLCO, which showed the most prevalent alteration in previous studies. Patients with COVID-19 underwent ICU follow-up clinic evaluation by a multidisciplinary evaluation that included physicians, physical therapists, psychologists, and nutritionists. Pulmonary function tests were performed by the physical therapist and evaluated by the pulmonologist. More elaborate tests, including DLCO, were outside the scope of this evaluation. The hospital's pulmonology service referred all patients with abnormal PFT results for evaluation and follow-up. Our results support that patients with severe COVID-19 need post-discharge care. Further research clarifying the pathophysiological mechanisms of lung injury may result in individualized pharmacological interventions and physical rehabilitation. Due to the large number of patients who are currently suffering from late complications from COVID-19, it would be of great importance that these patients could benefit from specific rehabilitation centers that would allow a multidisciplinary approach with the core of the pulmonologist and the respiratory physiotherapist but with access the various areas of health care to address this systemic disease.
Our findings showed that a significant proportion of patients discharged from the hospital after treatment for severe COVID-19 present pulmonary functional alterations at least 3 months after discharge. Moreover, these alterations affect the physical functional capacity and quality of life. Studies with larger populations and longer follow-ups are necessary to improve the knowledge about the health consequences of COVID-19.
Ethics approval and consent to participate
The trial protocol was approved by the Research Ethics Committee of the Hospital Sao Domingos (Number 5.403.663) in May 12, 2022 and registered in clinical trials. Gov (NCT05249842), February 22, 2022.
Consent for publication
Not applicable
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request
Competing interests
None declared
Funding
None declared
Authors' contributions
Conceptualization (JHCFL, FSTN, SCCS, LF, JRAA); Methodology (JHCLF, SCCS, GBT, LF, JRAA) Data collection and analysis (JHCLF, GBT, FSTN, AMCS); Original draft preparation (JHCLF, FSTN, SCCS, LF, AMSC); Supervision (JRAA); All authors have read and approved the manuscript.
Not applicable
Trial registration
The trial protocol was approved by the Research Ethics Committee of the Hospital Sao Domingos (Number 5.403.663) in May 12, 2022 and registered in clinical trials. Gov (NCT05249842), February 22, 2022.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Filho JHDCL, Tavares GB, Neto FDST, Souza SCDC, Freitas HL, Cruz AMDS (2024) Respiratory Function at 3 Months after Hospital Discharge in Critically Ill Patients with COVID-19. J Clin Trials. 14:554.
Received: 19-Jan-2024, Manuscript No. JCTR-24-29255; Editor assigned: 22-Jan-2024, Pre QC No. JCTR-24-29255(PQ); Reviewed: 05-Feb-2024, QC No. JCTR-24-29255; Revised: 12-Feb-2024, Manuscript No. JCTR-24-29255(R); Published: 19-Feb-2024 , DOI: 10.35248/2167-0870.24.14.554
Copyright: © 2024 Filho JHDCL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.