Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2014) Volume 5, Issue 5
Phenols are included in the list of potential toxins for motor neurons. Thus, fast and selective method for removal of phenols has been developed. The method was based upon the extraction of iron (III) phenolate complex anions [Fe(phenolate)6]3- by tri-n-butylphosphine (TBP)-plasticized iron(III) immobilized polyurethane foam (PUF) from the test aqueous solution adjusted to pH≈1 followed by subsequent formation of ternary ion association complex of these species with the protonated urethane and/or ether linkages on the PUF solid phase extractor (SPE). The rate of removal of phenols from the aqueous solution by TBP plasticized iron (III) immobilized PUF were studied in batch conditions employing a series of kinetic models. Initially, phenols uptake onto plasticized iron (III) treated PUF was fast followed by kinetically pseudo-second order rate equation with an overall rate constant (k) of 0.014 and 0.018 g (mg min)-1 for phenol and 2-chlorophenol (2-CP), respectively. Endothermic nature of phenols sorption is governed by the positive value of ΔH where as the negative values of ΔG dictate that the uptake of the analyte onto the sorbent is spontaneous phenomena. The positive values of ΔS for both phenols may be indicative of moderate sorption step of the complex anion [Fe(C6H5O)6]3- and ordering of ionic charges with a compensatory disordering of the sorbed species onto the sorbents. PUF sorbent provides efficient removal of phenols traces from various water samples.
Keywords: Phenols; Retention; Pseudo-second order model; Thermodynamic; Polyurethane foam
Phenolic compounds present in the aquatic environment as a result of their industrial applications such as a production of plastics, dyes, drugs, pesticides, anti-oxidants, paper and petrochemical [1]. The toxicity and unpleasant organoleptic properties of phenols at trace and ultra-trace concentrations in water and fish have been reported by the US Environmental Protection Agency (EPA) [2,3]. Relatively high concentrations have been measured in coastal seawater (up to 1500 ng L-1) for both 2,4-dichlorophenol (DCP), and penta chlorophenol (PCP) (PCP) [3,4]. Gao et al. [5] have reported the median levels of 5, 2, and 50 ng L-1for 2,4-DCP, 2,4,6-TCP and PCP, respectively, in China’s Rivers.
Numerous analytical techniques have been reported for determination of different phenolic compounds [6-10,11]. Simultaneous determination of 14 chlorophenols (CPs) and chloroanisoles (CAs) in wine samples have been carried out using stir bar sorptive extraction (SBSE) followed by gas chromatography-mass spectrometry (GCMS) [11]. Chlorophenols have been isolated from aquatic media and analyzed by high performance liquid chromatography with ultraviolet detection (HPLC-UV) [12-14]. However, none of these combinations can achieve the quantification limits required for direct determination of phenols in drinking water, resulting in a necessity for a preconcentration step. Separation and enrichment techniques such as liquid-liquid extraction (LLE) [15], solid phase extraction (SPE) [16- 23] and cloud point extraction (CPE) [24] have been widely used as a sample pretreatment step. Among these techniques, SPE has been used most frequently due to the availability, easy recovery of the solid phase and the high preconcentration factors that can be achieved [25].
PUF is a good sorbent material in SPE and it has been the subject of several review articles [15]. Recent years have seen an upsurge of interest for developing low cost, rapid and sensitive analytical methods for preconcentration and removal and subsequent determination of phenols in large sample volumes [7-11,26,27]. Thus, the work presented in this article is focused on studying the kinetics and thermodynamic characteristics and the most probable retention mechanism of the separation of some selected phenols onto PUF immobilized with iron (III).
Reagents and materials
All chemicals used were of analytical reagent grade and were used as received. All glassware’s were rinsed with doubly deionized water prior to use. Commercial white sheets of open cell PUF (polyether type) were purchased from a local market in Jeddah City, Saudi Arabia. Foam cubes of approximately 1.0 cm3 were cut from the PUF sheets and were washed and dried as reported [26]. Phenol and 2-CP were purchased from BDH (Poole, England). A standard stock solution (1000 μg mL-1) of each phenol was prepared in ultra-pure water in presence of few drops of ethanol. More diluted solutions (50-100 μg mL-1) of each phenol were prepared by appropriate dilution of the stock solutions with water. BDH Tri-n-butylphosphine (Poole, England) (0.01-0.05% v/v) was prepared in water in the presence of drops of ethanol. A series of Britton-Rrobinson (B-R) buffer solutions was prepared as reported [28].
Apparatus
A Perkin-Elmer UV-Vis spectrophotometer (Lambda 25, USA) with 10 mm (path width) was used for recording the electronic spectra of phenolic compounds. A Corporation Precision Scientific mechanical shaker (Chicago, CH, USA) was used. Deionized water was obtained from Milli-Q Waters Plus system (Milford, MA, USA). A Thermo Fisher Scientific Orion model 720 pH Meter (Milford, MA, USA) were also used.
Preparation of iron (III)-immobilized PUF
Accurate weights (0.2 g) of PUF cubes were shaken with 200 mL of different concentrations (0.01-0.05% m/v) of FeCl3.6H2O with efficient stirring for 30 min. The cubes were separated out, pressed between two sheets of filter papers and finally dried [27,28]. The amount of iron (III) retained onto the PUF was caculated from the difference between iron (III) before (C0) and after (C) shaking employing the following equation:
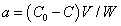 (1)
(1)
Where, V is the volume of solution in (mL) and W is the mass (g) of PUF sorbent. Plasticized iron (III)-immobilized PUF cubes were prepared by shaking iron (III) treated PUF with different concentrations of TBP (0.01-0.1% v/v) with efficient stirring for 30 min. The foam cubes were then treated as reportd [27].
Recommended extraction procedures
In a series of conical flasks (250 mL), an accurate weight of the unplasticized and plasticized iron (III)-immobilized PUF cubes (0.2 ± 0.002g) were equilibrated with 100-200 mL of an aqueous solution containing 50-100 μg mL-1 of the phenolic compounds. The solution pH was adjusted at pH ≈1. The solutions were shaken for 2 hours at 25 ± 1°C on a mechanical shaker. After phase separation, the aqueous phase was separated out by decantation and the amount of the phenols remained in the aqueous phase was determined by measuring the absorbance of the solution before and after extraction at λmax against a reagent blank [27,29]. Phenols retained on the PUF cubes were then calculated from the difference between the absorbance of the aqueous phase before (Ab) and after extraction (Af). The sorption percentage (%E), the amount of phenol retained at equilibrium (qe) and the distribution ratio (D) of the phenol uptake were calculated as reported [29]. Following these procedures, the influence of shaking time and temperature on retention of the target analytes by the PUF sorbents was fully studied. All experiments were carried out at least in triplicate and the results are the average of three independent measurements and the precision in most cases was ± 2%.
Solid PUF concentrates inorganic and organic substances from different media by phase distribution mechanism rather than adsorption [29,30]. The membrane-like structure of PUF, together with efficient aero- and hydrodynamic characteristics offer high concentrating ability and high flow rate compared to other solid supports [30]. The nonselective sorption characteristic of the PUF has been rendered more selective by controlling the experimental conditions. Preliminary study has shown that, on shaking plasticized TBP iron (III)-immobilized PUF individually with the aqueous solutions of phenols considerable amount of the analyte retained in the PUF in a short time. Hence, a detailed study was performed to assign the retention profile, kinetics, thermodynamics and the most probable sorption mechanism of phenols from the aqueous solutions by the proposed solid sorbent.
Retention profile of phenols from aqueous solution onto iron (III)-treated PUF
Iron (ІІІ) forms orange-red colored complexes with phenols in aqueous acidic solutions [31]. Thus, the sorption profile of aqueous solutions of phenols at different pH by iron (III)-loaded PUF was studied after 1h shaking time. After equilibrium, the amount of phenolic species in the aqueous phase was determined photometrically [31]. The %E and the D phenols sorption onto the PUF decreased markedly on increasing pH and reached maximum at pH ≈1 (Figure 1). At pH >1, the sorption of iron (III)-treated PUF towards phenols decreased markedly. This behavior is most likely attributed to the deprotonation of the phenolic species i.e. formation of polar phenolate species, absence of protonated ether oxygen (-CH2-O-CH2-) and/or urethane nitrogen (-NH-COO-) of treated iron (III) PUF, instability and/ or hydrolysis or incomplete extraction of the produced iron (III) phenolate complex and PUF solid sorbent.
The retention of phenols in strong acidic media is most likely attributed to the protonation of ether and/or urethane linkages available in PUF sorbent. This effect enhanced the retention of iron (III) phenolate complex anions on PUF via formation of a ternary ion associate with the protonated form of the PUF sorbent. Thus, in the extraction media of pH <1, the interaction of the complexed iron (III) phenolate complex speciesis easily proceeded with the protonated ether group of the PUF. Based on these data and the results reported on the retention of AuCl4 - by PUF [29], a sorption mechanism involving a weak base anion ion exchange and/or solvent extraction of iron (III) phenolate complex [Fe(phenolate)6]3- by the protonated ether (-CH2-HO+-CH2-) oxygen linkage of the PUF as a ternary complex ion associate is most likely proceeded [26-30] as follows:
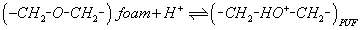 (2)
(2)
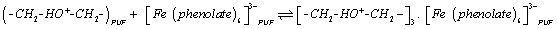 (3)
(3)
The influence of the plasticizer TBP (0.01-0.05% v/v) on phenols uptake from the aqueous solutions onto the iron (III)-loaded PUF was studied. Phenols sorption onto the sorbent dramatically increased (D =1.6 × 104mL g-1) in the presence of TBP (0.01% v/v). The fact, TBP enhanced the swollen character of the sorbent and decreases the glass transition temperature of the sorbent [30]. This effect enhanced the diffusion of the species within the PUF membranes [30]. These results suggested the possible use of the TBP plasticized iron (III)-loaded PUF in packed column for enrichment and subsequent analysis of phenols from large volumes of aqueous solutions. The retained phenols species were successfully recovered with NaOH (10 ml, 0.01 mol dm-3) and finally analyzed [31].
Kinetic behaviour of phenols
The influence of shaking time (0-120 min) on the uptake of the phenols from the aqueous acidic media at pH≤1 was investigated. The sorption of the phenols onto the iron (III)-immobilized PUF sorbent was fast and reached equilibrium after 60 min shaking time. This conclusion was supported by calculating the half-life time (t1/2) of phenols sorption from the aqueous solutions onto the PUF sorbents (Figure 2). The values of t1/2 were calculated from the plot of -log Ab-Af/ Ab versus time as shown in Figure 2. The values of t1/2 were found to be1.2 ± 0.04 min for phenol and 1.05 ± 0.008 min for 2-CP. Thus, gel diffusion is not only the rate-controlling step for iron (III)-loaded PUF as in the case of common ion exchange resins [29,30] and the kinetic of phenols sorption onto the sorbent depends on film and intraparticle diffusion. Thus, the results were subjected to a number of kinetic models.
Weber-Morris model [32] can be expressed as follows:
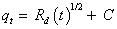 (4)
(4)
Where Rd is the rate constant of intraparticle transport (mg g-1 min- 1/2), qt is the sorbed phenol concentration (mg g-1) at time t and C (mg g-1) is the intercept. The plots of qt versus time were slightly linear with R2=0.9438 and 0.9652 for Phenol and 2-CP, respectively, in the initial stage (Figure 3) up to 16 ± 1 min and 2-CP deviate on increasing the shaking time in the initial stage. The change in the slope is most likely due to the existence of different pore size [29]. The plot does not pass through the origin, thus it can be assumed that a boundary layer effect occurs at a given degree and intraparticle diffusion process is not the unique rate controlling step [33].
The results were further subjected to Lagergren model for pseudofirst order [34]:
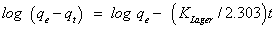 (5)
(5)
Where, qe is the amount of phenol sorbed at equilibrium per unit mass of sorbent (mg g-1) and KLager is the first order overall rate constant of pseudo-first order kinetic for the retention process per min and t is the time in min. The values of Lagergren parameters KLager and qe calculated from the linear plot of log (qe-qt) vs. time (Figure 4) are summarized in Table 1. Because of the large difference between the calculated and experimental values of qe [35], the order of the sorption process changed after elapsed time and the experimental data could not be adjusted to pseudo-first order equation (4). This trend could indicate that, the first stage of the retention process (external transport of phenol from the bulk solution to sorbate surface) follows pseudofirst order kinetics while the posterior processes (film diffusion and intraparticle diffusion) do not follow the same order. Thus, the results of phenols uptake were subjected to the pseudo-second order kinetic model [35,36]. This model can be expressed by the following equation:
| Compound | qe (mg g-1) experimental | First-order kinetic model(Lagergren model) | Second-order kinetic model | ||||
|---|---|---|---|---|---|---|---|
| K1 (min-1) | qe | R2 | K2×10-2g(mg.min)-1 | qe | R2 | ||
| Phenol | 73.916 | 0.108 | 55.39 | 0.9727 | 1.40 | 71.43 | 0.9955 |
| 2-CP | 51.45 | 0.021 | 4.85 | 0.9759 | 1.80 | 51.54 | 0.9992 |
Table 1: Kinetic parameters for pseudo first-order and second-order kinetic models for sorption of phenol and 2-CP onto TBP plasticized iron (III)-immobilized PUF at 25 ± 1°C.
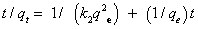 (6)
(6)
Wherek2 (g mg-1 min-1) is the pseudo second-order rate constant, The plots of t/qt versus t for both phenols were linear (Figure 4). Kinetic data obtained gave very good fit with the pseudo-second order model as shown by the excellent correlation coefficients R2=0.996-0.999 compared to Lagergren model 0.973-0.976 (Table 1). The secondorder rate constant (k2), equilibrium capacity (qe), and the correlation coefficient (R2) for the tested phenols are summarized in Table 1. The pseudo-second order constants (k2) for phenol and 2-CP were 1.40 and 4.85 (g mg-1.min-1), respectively. Based on analyzing the kinetic data, it can be concluded that, the first step of the process (mass transport from solution until sorbent) followed pseudo-first order kinetics and at the early stage of extraction and the whole sorption process is governed by a pseudo-second-order kinetics [35,36].Thus, a chemi-sorption reaction is most likely predominate in the rate-controlling step [37,38]. The adsorption capacity calculated for phenol and 2-CP were 55.39 and 4.85 mg g-1, respectively.
Thermodynamic characteristics
A thermodynamic characteristics of sorption of phenols from the aqueous solution by plasticized TBP- and un plasticized iron (III)- treated PUF was critically performed over a wide range of temperature (288-338 K). The thermodynamic parameters e.g. ΔH, ΔS and ΔG) were evaluated using the following equations [39]:
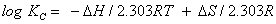 (7)
(7)
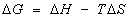 (8)
(8)
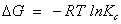 (9)
(9)
Where ΔH, ΔS, ΔG, T and R are the enthalpy, entropy, Gibbs free energy changes, temperature in Kelvin, and the gas constant (8.314 J K-1 mol-1), respectively. KC is the equilibrium constant depending on the fractional attainment (Fe) of the sorption process. The KC for retention of phenols at equilibrium onto the sorbent was calculated for each temperature employing the following equation:
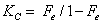 (10)
(10)
The plot of ln KC versus 1000/T for phenol retention onto plasticized and un plasticized iron (III)-immobilized PUF were linear (Figure 5) over the temperatures range (288-338 K). Similar trend was also achieved for 2-CP. The equilibrium constant increased on decreasing temperature revealing that the retention process of [Fe(C6H5O)6]3- species onto the PUF sorbent is an endothermic process for both phenols. The numerical values of ΔH, ΔS and ΔG for phenol and 2-CP retention calculated from the slopes and intercepts of the linear plots of ln KC against 1000/T are summarized in Table 2.
| Compound | ∆H**, Jmol-1 | ∆S, J mol-1 K-1 | ∆G, KJmol-1 | R2 |
|---|---|---|---|---|
| Phenol | 76.75 (78.58) |
233.3 (231.3) |
-69.45 (-68.85) |
0.9938 (0.9568) |
| 2-CP | 38.64 (47.8) |
104.51 (120.8) |
-31.11 (-35.95) |
0.9162 (0.9682) |
*The values of thermodynamic parameters using plasticized iron(Ш)-immobilized PUF are given in parentheses.
**The calculated values of ΔH from Vant,hoff equation for phenol and 2-CP retention onto unplasticized- and plasticized iron (III) immobilized PUF were found 75.55 (78.72) and 38.7 (48.33) Jmol-1, respectively.
Table 2: Numerical thermodynamic parameters (ΔH, ΔS and ΔG) for the tested phenols onto unplasticized and TBP plasticized iron (III)-immobilized PUF*.
According to Van`t Hoff equation [38], the distribution coefficient (D) of the phenol retention is correlated with temperature according to the following expression:
 (11)
(11)
Where, C is constant. The plots of log D versus 1000/T for phenol retention onto plasticized and unplasticized iron (III)-immobilized PUF were linear (Figure 6). Similar trend was also achieved from 2-CP. The computed values of ΔH for the sorption of both phenols from the slopes of the plots are summarized in Table 2. These results are quite close to the values computed from equations 7 and 8.
The positive values of ΔH and the values of D and KC reflect the endothermic behavior of phenols retention and the non-electrostatics bonding formation between the sorbent and the sorbate. The negative values of ΔG (Table 2) imply the spontaneous and physical sorption nature of the phenols. The positive values of ΔS for both phenols may be indicative of moderate sorption step of the complex anion [Fe(C6H5O)6]3-and ordering of ionic charges with a compensatory disordering of the sorbed species onto the sorbents. Thus, the freedom of [Fe(C6H5O)6]3-motion is less restricted in the PUF membrane than in solution and the sorption process involves a decrease in the free energy. Thus, the physical structure of the PUF membrane may be changing and affecting the strength of the intermolecular interactions between PUF membrane and the [Fe(C6H5O)6]3-species. The high temperature may also make the membrane matrix become more structured and does not affect the ability of the polar segments to engage in unstable hydrogen bonding with [Fe(C6H5O)6]3-species resulting in higher extraction. The energy of urethane nitrogen and/or ether-oxygen sites of the PUF provided by raising the temperature on the phenol and 2-CP uptake by the PUF sorbent is most likely maximizes the possible interaction between the active sites of the PUF and the bulky complex anion [Fe(C6H5O)6]3- resulting in a higher sorption related to "Solvent extraction" and an added component for "surface adsorption". Thus, the dual mode sorption mechanism of these analytes by the used PUF sorbent seems a more probable model for tested compounds.
Iron (III)-immobilized PUF can be used as an effective sorbent for the removal of phenols from water samples in strong acidic conditions. The formation of non-ionized species of phenols and the formation of ternary complex ion associate between the complex anion of iron (III) with phenols i.e. [Fe(phenolate)6]3-and the protonated PUF results in a better extraction. The retention kinetics followed a pseudo-second order model and according to Weber-Morris model, an intraparticle diffusion process participates in the adsorption rate. The amount of retained phenols increased with the increase of temperature, indicating that, the adsorption is an endothermic process. Work is still continuing for increasing the number of phenols that can be retained, separated out and simultaneously determined by the proposed method. Further studies will involve an investigation of these extractions for a wider range of organic compounds in order to obtain a clearer view of the extraction mechanism.