Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2021)
The current worldwide COVID-19 pandemic caused by SARS-CoV2 has generated an urgency to understand the implications of this disease in the eye. Possible retinal involvement by SARS-CoV2 infection has been a topic of heated debate.
Objective: To determine whether retinal abnormalities can be identified on Optical Computed Tomography (OCT) in convalescent patients following COVID-19 infection.
Methodology: This is a prospective, case controlled study that recruited COVID-19 patients who were admitted to the United Christian Hospital Hong Kong, China. At 2 months post-recovery, patients’ visual acuity, refraction were measured. Spectraldomain OCT of the macula to retinal nerve fiber layer and enhanced depth imaging were performed. Age-matched and refraction-matched healthy individuals that were not infected with COVID-19 were enrolled as controls. Qualitative and quantitative assessments of retinal abnormalities on OCT and retinal and choroidal layer thickness were done.
Results: 20 subjects (40 eyes) with COVID-19 and 25 (50 eyes) healthy controls were enrolled. Structural OCT abnormalities were observed in 24% of control eyes and in 25% of COVID-19 subjects. No differences were observed between the post- COVID-19 cohort and the healthy controls for any qualitative retinal abnormalities or in any quantitative features including retinal volume, choroidal thickness, retinal layer thicknesses in various macular regions, and peripapillary nerve fiber layer thickness.
Conclusion: Following full recovery from symptomatic COVID-19 infection, no significant abnormalities were evident on structural OCT as compared to controls. Although long-term damage to the retina appears to be uncommon after COVID-19 infection, this study provides valuable insight into the recovery process after COVID-19 and provides potential differentiating retinal features that should be considered in future studies involving a larger population.
COVID-19; SARS-CoV2; Optical coherence tomography; Retina; Macula; Recovery; Prospective study
The current coronavirus disease 2019 (COVID-19) pandemic has taken the lives of more than 555,314 individuals in the United States and a staggering 2.7 million lives worldwide as of March 2021 [1]. This has placed a tremendous strain on the healthcare system worldwide and calls for urgent scientific efforts to rapidly understand the pathobiology of this disease. COVID-19 is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2 a family member of coronaviruses that can cause acute respiratory symptoms, multisystem complications and death [2]. Current research data suggests that SARS-CoV2’s primary mode of transmission is through the respiratory tract and various reports point towards the eye as another possible route of entry [3-5]. Even after recovery and clearance of the virus from the body, many survivors of COVID-19 have reported lingering symptoms that spans many organ systems [6,7].
Recent studies have corroborated and demonstrated that COVID-19 has ocular manifestations that include conjunctivitis manifested by conjunctival hyperemia, chemosis, epiphora, and/ or increased secretions [8-10]. These findings have primarily focused on manifestations on the ocular surface, but there is no consensus as to manifestations in the retina, which have sparked an international debate in the ophthalmic community [11-16]. Furthermore, SARS-CoV2 has been found on the conjunctiva in individuals without overt conjunctival symptoms [17]. These findings suggest the need to look beyond the ocular surface and explore whether SARS-CoV2 is able to affect the eye in the posterior segment. The works of Marinho suggested that SARSCoV2 causes hyper-reflective foci within the ganglion cell layer and inner plexiform [18]. However, the identity of these lesions (whether they may be normal retinal blood vessels) and their significance remains to be elucidated.
A review of literature has shown evidence that other viruses can infect the central nervous system and lead to retinal manifestaions [19-21]. This is primarily due to the viral inflammatory response which can affect the vasculature and lead to occlusive disease. A recent study has linked COVID-19 to thrombotic complications which raised the possibility of an impact on the retinal vasculature [22]. Optical Coherence Tomography (OCT) is a non-invasive retinal imaging technique at subcellular level. As the retina is an extension of the CNS, OCT has been used for monitoring the progression of neurological diseases such as Alzheimer’s disease and multiple sclerosis [23-27]. Similarly, in this study we aimed to investigate the potential impact of COVID-19 infection on retinal morphology in patients that were acutely ill but made full recovery.
This is a prospective case-control study. Patients with proven COVID19 disease admitted to United Christian Hospital (UCH) of Hong Kong between March 28th 2020 to April 9th 2020 were recruited. UCH serves the eastern region of Kowloon peninsula of the Hong Kong Special Administrative Region, with a catchment population of over 1,155,000. This study was conducted in accordance with the Declaration of Helsinki and the study protocol was approved by the Institutional Review Board of the Kowloon Central/Kowloon East Cluster of Hospital Authority (Reference number: (KC/KE)-20-0081/ER-4) and the University of California Los Angeles. Informed consent was obtained from all patients.
Inclusion and exclusion criteria
To be included in this study, patients had to be aged 18 years or older; have a history of COVID-19 infection with confirmation of SARS-CoV2 by real-time reverse transcriptase polymerase chain reaction (RT-PCR) from respiratory or gastrointestinal samples; followed by full recovery from COVID-19 within two months after the initial infection after the initial infection. Exclusion criteria included patients with a previous known history of any retinal diseases, with the exception of myopia. Age-matched healthy individuals without COVID-19 and without a known history of retinal disease were recruited into the control group.
Data collection and imaging
A total of 45 patients (20 COVID19 patients and 25 controls) were recruited. Demographical data such as gender, age and spherical equivalent (SE), visual acuity were recorded. Patient’s ocular and systemic symptoms were documented. Chest X-Ray image findings during hospitalization were analyzed for patients in the COVID19 group. For the disease group, severity of COVID19 disease was classified as mild, moderate, severe and critical based on symptoms, clinical findings and CXR results, as defined by guideline of PC-NCP4 [28].
OCT images were captured using the Heidelberg Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) for COVID19 patients during their convalescent phase (2 to 3 months post hospital discharge). The imaging protocol consisted of the following: Infrared reflectance image–55 degrees, blue-light fundus auto fluorescence image–55 degree, Spectral is OCT using a 25 horizontal line protocol (6*6 mm area) each consisting of 1.024 A-scans per line–30 degree, Full Depth Imagining (FDI) 1 horizontal, 1 vertical and 2 oblique scan lines–30 degree and Enhanced Depth Imaging (EDI) 1 horizontal scan line–30 degree. In addition, an optic disk image with a scan circle diameter of 3.45 mm was positioned manually and centered on the center of the optic disc.
All imaging data were transmitted in a de-identified fashion to the Doheny Image Reading Center for masked analysis by certified OCT graders. OCT volumes were qualitatively assessed for any abnormalities, including abnormalities in layer continuity, reflectivity, and thickness. In addition, the OCT was analyzed quantitatively for several parameters including: mean retinal thickness for each of the 9 map sectors as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS), macular volume, peripapillary retina nerve fiber layer thickness, which included 6 regional subfields (nasal, inferonasal, inferotemporal, temporal, superotemporal, and superonasal) as well as global (central) and mean subfoveal choroidal thickness.
Data analysis
OCT quantitative features were transformed using a generalized logarithm transformation (glog) and scaled using a mean-centered scale divided by the standard deviation of each feature. To measure variability within samples a multivariable dimension-reduction analysis was implemented. Principal component analysis (PCA) plots were plotted using the first and second PC accounting for 65.8% of total variance, with the 95% confidence region displayed in the plot (green=healthy, red=COVID-19). Loading plots were generated using the first and second PC. A categorical bilinear factor model analysis was implemented to determine categorical distribution between the two groups. Similarly, the partial least squares discriminant analysis (PLS-DA) was plotted using the first and second PC, displaying the 95% confidence region of the two groups and loading plots used the first and second PC. Parameters for the volcano plot analysis included a fold change threshold (FC)=2.0, a comparison ratio=COVID-19/Healthy, p-value threshold=0.05, and an assumption of equal variance within each group. Y-axis was displayed as -log10 (p-value) and x-axis as log2 (FC). T-test analysis and Fisher’s exact test used an alpha value of 0.05 as the cutoff.
Patient demographics
A total of 45 subjects were recruited, with 20 in the COVID-19 group and 25 in the healthy group. There was no statistically significant difference in the gender distribution, age, pin-hole visual acuity and refractive error between the two groups (Table 1).
| Population characteristics | Healthy | COVID-19 | P value |
|---|---|---|---|
| Sample size, no. | 25 (50 eyes) | 20 (40 eyes) | NA |
| Gender (male/female) | 10/15 | 11/9 | 0.377a |
| Age (yrs) | 48.32 ± 14.39 | 44.55 ±18.03 | 0.439b |
| Pinhole visual acuity (Decimal) | 0.87± 0.16 | 0.89 ± 0.15 | 0.470b |
| Refractive error (D) | -1.67 ± 2.32 | -1.53 ± 2.63 | 0.840c |
Mean ± standard deviation; D = Diopter; NA = not available, a Fisher’s exact test; b T-test; c GEE
Table 1: Patient demographics.
The mean age of the disease group and the control group was 44.6 years old (range 20-72) and 48.3 years old (range 15-72) respectively. There was a mixture of ethnic groups within the disease group, although the majority (90%) were Chinese in origin, 0.5% were Southeast Asians and 0.5% were Caucasians. For the control group, 100% of subjects were Chinese. For COVID19 disease severity, 65% had mild disease with minimal clinical symptoms and clear chest imaging, while 20% had moderate disease (positive respiratory symptoms with positive chest imaging of pneumonia) and 15% had severe disease (with tachypnoea or desaturation in addition). No patient in this study had COVID19 disease of critical severity in addition to moderate disease criteria.
Ocular symptoms
None of the healthy control subjects reported any visual symptoms. Among the COVID-19 subjects, 4 (20%) reported symptoms of ocular surface inflammation within 2 weeks prior to admission. These included epiphora (15%), itchiness (20%), ocular pain and foreign body sensation (10%), blurring of vision (10%) and redness (5%). One patient (5%) had overt bilateral transient conjunctivitis which resolved spontaneously within the first week of admission.
OCT findings
Overall, 24 % of healthy eyes and 25% of COVID-19 eyes demonstrated some evidence of an abnormality on qualitative inspection of the OCT (Table 2, Figure 1), though none of the abnormalities were deemed to be of visual significance. No significant differences were observed in terms of the frequency or distribution of abnormalities. Specifically, we did not observe any cases with focal thinning of inner or middle retina layers to suggest ischemic injury.
| Feature | Healthy (50 eyes) |
COVID-19 (40 eyes) |
P valuea |
|---|---|---|---|
| Vitreomacular adhesion | 5 | 2 | 0.456 |
| ERM | 2 | 0 | 0.501 |
| Macular hole-lamellar/pseudohole | 1 | 1 | 1 |
| Intraretinal cystoid spaces/fluid | 1 | 0 | 1 |
| Intraretinal hyperreflective | 2 | 2 | 1 |
| Peripapillary RNFL loss | 2 | 4 | 0.401 |
| Peripapillary atrophy | 0 | 3 | 0.084 |
| Drusen/drusenoid PED | 3 | 5 | 0.458 |
| PED-Serous | 1 | 2 | 0.583 |
| Subretinal fluid | 1 | 1 | 1 |
| Subretinal tissue | 0 | 2 | 0.195 |
| Focal choroidal excavation | 2 | 0 | 0.501 |
| Myelinated nerve fiber layer | 1 | 0 | 1 |
| Myopia | 0 | 2 | 0.195 |
ERM = epiretinal membranes; RNFL = retinal nerve fiber layer; PED = pigment epithelial detachment, a Fisher’s exact test.
Table 2: Summarized OCT qualitative features in all patients.
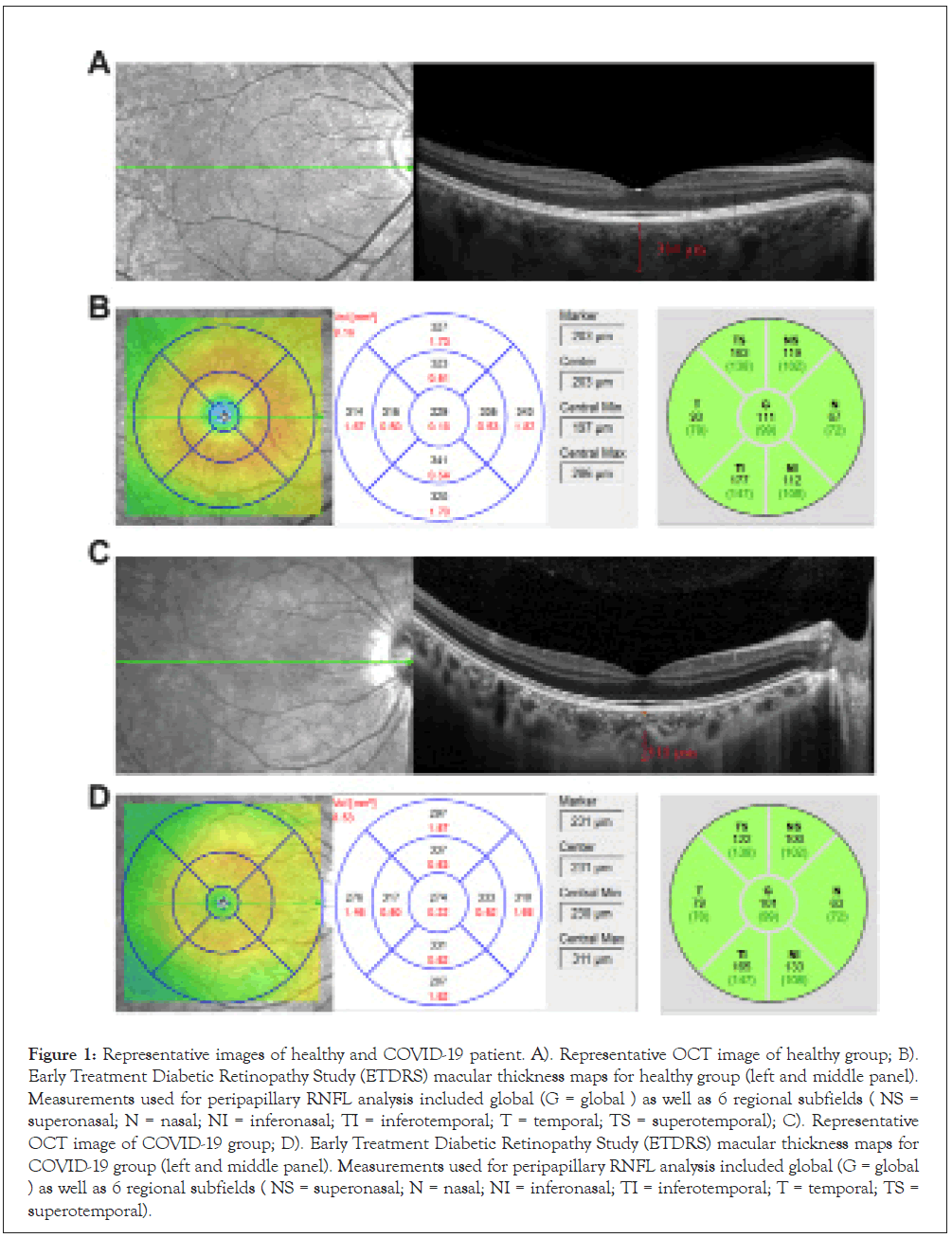
Figure 1: Representative images of healthy and COVID-19 patient. A). Representative OCT image of healthy group; B). Early Treatment Diabetic Retinopathy Study (ETDRS) macular thickness maps for healthy group (left and middle panel). Measurements used for peripapillary RNFL analysis included global (G = global ) as well as 6 regional subfields ( NS = superonasal; N = nasal; NI = inferonasal; TI = inferotemporal; T = temporal; TS = superotemporal); C). Representative OCT image of COVID-19 group; D). Early Treatment Diabetic Retinopathy Study (ETDRS) macular thickness maps for COVID-19 group (left and middle panel). Measurements used for peripapillary RNFL analysis included global (G = global ) as well as 6 regional subfields ( NS = superonasal; N = nasal; NI = inferonasal; TI = inferotemporal; T = temporal; TS = superotemporal).
Principal component analysis demonstrated a substantial overlap in the 95% confidence region between the two groups (COVID-19 and Healthy) which indicates that these groups have similar variances (Figure 2A, left panel). Further analysis via the partial least squares discriminant analysis also demonstrated a similar overlap, suggesting that these groups do not separate into two different categories and that there are no differences between the two groups (Figure 2B, left panel). Loading plot analysis illustrated the quantitative features that contribute to the majority of variance in the PCA and PLS-DA. Macular volume contributed the majority of variation to the first principal component and peripapillary nerve fiber layer thickness in the superonasal region to the second principal component in the PCA (Figure 2A, right panel). Peripapillary nerve fiber layer superonasal region contributed the majority of the variation to the first principal component and peripapillary nerve fiber layer inferotemporal region to the second principal component in the PLS-DA (Figure 2B, right panel).
T-test analysis demonstrated that most quantitative features did not reach statistical significance (p<0.05) with the exception of the retinal outer temporal quadrant region (Table 3). Although this quantitative feature reached statistical significance, volcano plot analysis demonstrated that this quantitative feature was below a fold change ratio of 1.0 (Table 3).
| Region | Healthy (50 eyes) |
COVID-19 (38 eyes) | P value a | -log10(p)b | log2(FC) |
|---|---|---|---|---|---|
| Subfoveal choroidal thickness | 293.6 ± 93.6 | 306.3 ± 111.1 | 0.666 | N.S. | N.S. |
| Marker center | 217.0 ± 18.80 | 221.7 ± 18.8 | 0.400 | N.S. | N.S. |
| Center (0.5 mm radius) |
260.1 ± 21.85 | 263.0 ± 20.45 | 0.649 | N.S. | N.S. |
| Inner ring (1.5 mm radius) | |||||
| Superior | 341.7 ± 16.72 | 344.1 ± 16.83 | 0.625 | N.S. | N.S. |
| Inferior | 337.9 ± 15.17 | 337.7 ± 15.99 | 0.973 | N.S. | N.S. |
| Temporal | 329.2 ± 15.97 | 327.6 ± 16.09 | 0.731 | N.S. | N.S. |
| Nasal | 345.2 ± 15.54 | 345.2 ± 16.70 | 0.572 | N.S. | N.S. |
| Outer ring (3 mm radius) | |||||
| Superior | 301.1 ± 13.75 | 305.1 ± 15.44 | 0.354 | N.S. | N.S. |
| Inferior | 288.7 ± 13.22 | 291.3 ± 14.29 | 0.508 | N.S. | N.S. |
| Temporal | 284.8 ± 11.39 | 290.4 ± 14.02 | 0.139 | 1.32 | N.S. |
| Nasal | 317.4 ± 15.26 | 319.9 ± 15.69 | 0.587 | N.S. | N.S. |
| Macular volume | 8.64 ± 0.36 | 8.72 ± 0.41 | 0.496 | N.S. | N.S. |
| Peripapillary RNFL | |||||
| Superonasal | 116.0 ± 23.74 | 112.2 ± 27.11 | 0.583 | N.S. | N.S. |
| Nasal | 65.04 ± 15.14 | 64.5 ± 18.66 | 0.904 | N.S. | N.S. |
| Inferonasal | 105.5 ± 20.47 | 112.1 ± 24.77 | 0.326 | N.S. | N.S. |
| Inferotemporal | 166.0 ± 21.95 | 156.4 ± 21.79 | 0.130 | N.S. | N.S. |
| Temporal | 87.60 ± 14.08 | 87.16 ± 21.01 | 0.933 | N.S. | N.S. |
| Superotemporal | 144.2 ± 19.92 | 147.3 ±21.60 | 0.566 | N.S. | N.S. |
| Global | 104.4 ± 10.66 | 103.9 ±10.86 | 0.867 | N.S. | N.S. |
Thickness unit in microns (μm), volume unit in mm3, RNFL= retinal nerve fiber layer. a GEE, b T-test, N.S. = no significance
Table 3: Descriptive details of OCT quantitative features in all patients.
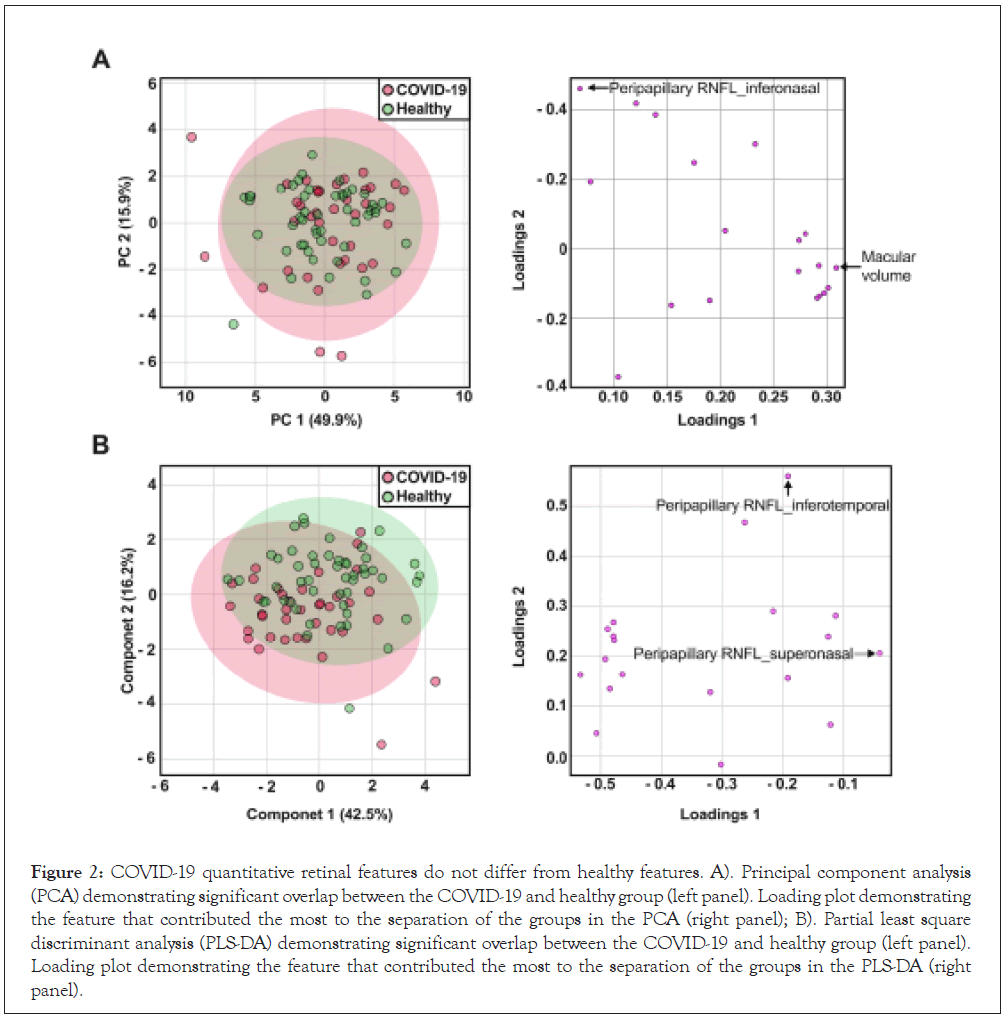
Figure 2: COVID-19 quantitative retinal features do not differ from healthy features. A). Principal component analysis (PCA) demonstrating significant overlap between the COVID-19 and healthy group (left panel). Loading plot demonstrating the feature that contributed the most to the separation of the groups in the PCA (right panel); B). Partial least square discriminant analysis (PLS-DA) demonstrating significant overlap between the COVID-19 and healthy group (left panel). Loading plot demonstrating the feature that contributed the most to the separation of the groups in the PLS-DA (right panel).
To date, the ocular pathobiology of COVID-19 remains largely unknown. Although ocular surface manifestations have been documented by different studies, intraocular injury, and in particular involvement of the posterior segment, remain a topic of debate. COVID-19 patients have been reported have been reported to show neurological symptoms in addition to respiratory symptoms. Furthermore, COVID-19 RNA has been detected in the retina and cerebrospinal fluid, suggesting a potential neuro-ophthalmic pathobiology [29,30]. Similarly, other viruses are known to directly and indirectly (via immunological or inflammatory pathways) cause neurological and retinal pathology [31,32]. The retina being an extension of the central nervous system may be susceptible to nerve damage by COVID-19. In this study we sought to investigate changes in retinal morphology associated with COVID-19 infection.
To our knowledge this is the first study to compare OCT features between fully recovered COVID-19 patients and healthy subjects. Our results demonstrate that qualitative and quantitative retinal features do not differ significantly between the two groups. Despite this lack of statistical difference, macular volume and peripapillary nerve fiber layer superonasal region were the two features that contributed the most to the separation of the groups in the PCA, while peripapillary nerve fiber layer superonasal region and peripapillary nerve fiber layer inferotemporal region contributed the most separation to the groups in the PLS-DA. Although not statistically significant, these quantitative features are the most likely to show differences between the two groups and have the potential for becoming features of interest in future studies. Further significance analysis revealed that the retinal outer temporal quadrant region was statistically significant, which would suggest a contribution to group separation. However, the impact this quantitative feature has on the dataset is miniscule given that its fold change impact was below 1.0. However, this feature should be considered as a potential candidate for investigation in future studies.
A recent study which scrutinized for the presence of retinal anomalies in COVID-19 infected patients determined that cotton wool spots (CWS) were a potentially relevant feature [33-35]. The presence of a single CWS is certainly not normal, but there are numerous potential causes of CWS. CWS, if relevant at all to COVID-19, might not be a direct manifestation of COVID-19 infection, but rather the result of a broader systemic immune response or as a result of treatments administered for COVID-19. It should be noted that we inspected carefully for the presence of focal inner or middle retinal thinning, which are telltale signs of previous nerve fiber layer (NFL) infarct or paracentral acute middle maculopathy (PAMM) respectively, but did not find such changes [36-38].
Pereira reported retinal abnormalities during severe COVID-19 infection, which were associated with the severity of infection. Another group focused on patients that were 14 days post hospital discharge and found similar anomalies. Moreover, the SERPICO-19 study demonstrated that retinal venous diameter appeared to directly correlate with the severity of infection. Although these findings point towards retinal abnormalities as being a feature of COVID-19, a common limitation of these studies was the lack of inclusion of a control group. One might assume this may be unnecessary when the control group is anticipated to be normal, however, in previous screening studies; we identified abnormalities on OCT in a high proportion of asymptomatic subjects [39]. Indeed, in the present study, an abnormality was identified on OCT in 24% of control eyes.
The lack of any significantly increased abnormalities as compared to control group suggest that the retinal anomalies reported during the course of COVID-19 infection might be specific to earlier phases of the disease process and may not be apparent once a patient makes a full recovery. This may be good news for COVID19 patients as our data suggest that there are no longterm consequences of SARS-CoV2 on the retina.
It is important to note that our study sample is relatively small and furthermore, the majority of our study subjects had mild to moderate COVID19 disease, thus patients with severe to critical disease are under-represented. It is possible that, it is possible that long-term retinal findings develop in some COVID19 patients, but this occurrence is relatively uncommon. That would not be surprising given the known heterogeneity in COVID-19 disease severity and individual patient’s susceptibility for complications. Nonetheless, the fact that longer-term retinal complications are uncommon is a useful finding. The extensive statistical analysis including PCA and PLS-DA used in this study give us confidence that significant retinal abnormalities were not present in this cohort. While larger confirmatory studies are clearly necessary, our findings suggest that structural OCT abnormalities are not common in individuals after full recovery from COVID-19 infection.
The authors would like to dedicate this paper to all staff of the Department of Ophthalmology, United Christian Hospital and Tseung Kwan O Hospital. The authors would like to express our appreciations to Mr. Wu Moon Raymond, Ms. Yu Ka Ka, Mr. Sze Man Fung Billy for their valuable contribution and expertise in retinal imaging and Mr. Wong Siu Hin Kelvin for his technical support to our study.
K.K.W.L., S.R.S. and S.S.L, conceptualization; S.S.L and L.M.Y, data acquisition and curation; Y.H. and F. C., grade OCT scan; Y.H. and S.S.L, formal analysis; K.K.W.L. and S.R.S., supervision; K.K.W.L. and S.R.S., funding acquisition; Y.H., validation; K.K.W.L. and S.R.S., principal investigator; S.S.L and Y.H., methodology; Y.H. and S.R.S., writing-original draft; K.K.W.L. and S.R.S., project administration; and Y.H., S.S.L, S.R.S. and K.K.W.L., writing: review and editing.
Citation: He Y, Liu S, Corvi F, Yeung T LM, Tso E YK, Sadda SR, et al. (2021) Retinal Findings on OCT in a COVID-19 Patient Cohort. J Clin Exp Ophthalmol. S13:002.
Received: 23-Mar-2021 Accepted: 09-Apr-2021 Published: 16-Apr-2021 , DOI: 10.35248/2155-9570.21.s13.002
Copyright: © 2021 He Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.