Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2015) Volume 6, Issue 2
Enzyme immobilization is a promising approach to reduce enzyme cost in lignocellulose-based biorefining. This paper describes the reusability of immobilized cellulases and examines hydrolysis of various components of lignocellulose and industrial lignocellulosic biomass when using immobilized cellulases. Two different commercial cellulases, previously denoted as Cellulases 1 (C1) and Cellulases 2 (C2), were separately immobilized on nonporous (S1) and porous (S2) silica. Enzyme immobilization was achieved using a simple, cheap, and safe absorption method that maintains high hydrolysis yields by creating a cellulosome-like environment. Here, we show that all immobilized cellulases could be reused for at least 4 cycles while maintaining ≥ 50% of their activity. In fact, systems containing immobilized C1 displayed >40% activity in the 6th cycle, regardless of the silica used. We obtained relatively high-retained enzyme activities when our immobilized cellulases were employed to hydrolyze cellophane paper (60%–78%), phosphoric acid swollen cellulose (72%–79%), a common component of hemicellulose (xylan; 62%– 84%), steam-exploded poplar (41%–62%), and waste office automation paper (34%–48%). Thus, the immobilized cellulase systems used in this study may be industrially feasible as they can be reused while maintaining relatively high levels of enzyme activities. Importantly, we also show that our immobilized cellulase systems can be applied to not only model substrates, but also to industrially produced lignocellulosic biomass.
Keywords: Cellulases; Cellulosome; Immobilization; Reusability; Stem-exploded poplar; Waste office automation (OA) paper; Hemicellulose; Amorphous cellulose; Hydrolysis
Biorefining is of growing interest to investors and the environmentally concerned public. Cellulose, the most abundant biomass component on earth, is of particular interest as a sustainable source of energy and bioproducts [1-4]. However, significant challenges must be overcome before lignocellulose can be realized as a sustainable and profitable resource. In particular, the cost of cellulosedegrading enzymes, cellulases, is a primary hurdle. Cellulases are enzyme complexes composed of hydrolytic and oxidative enzymes, which synergistically promote the degradation of cellulose to glucose [5]. One study examining biofuel production at second-generation ethanol plants determined that enzyme costs accounted for 32% of the total cost [6]. Currently, cellulosic ethanol production has a minimum fuel selling price (MFSP) of $3.27/gasoline gallon equivalent [7]. Not only are enzymes expensive and as of yet difficult to reuse, they are also short-lived, which makes their long-term storage difficult, further increasing costs and reducing industrial efficiency. Cellulosomes are multi-enzyme complexes found on the cell surface of many anaerobic bacteria and are involved in the hydrolysis of cellulosic and hemicellulosic material [8,9]. When fully assembled on the cell surface, cellulosomes are generally in the MDa size range [8]. The cellulosome is held together by a scaffoldin, which is a structural protein containing a varying number of cohesin domains that interact with dockerin domains [8,10]. The latter are present in a variety of hydrolytic enzymes, including cellulases, hemicellulases, pectinases, chitonases, glycosidases, and esterases [10]. Cellulosomes also contain carbohydrate-binding modules (CBM) that mediate binding to substrates, such as insoluble cellulose. It is believed that cellulosomes increase rates of biomass degradation by concentrating a variety of hydrolytic enzymes to a single site [8,9]. In several recombinant systems examined, the creation of a designer cellulosome led to increased hydrolytic activity, relative to corresponding free enzyme systems [11,12]. Previously, our group identified an efficient, simple, cheap, and safe cellulase immobilization method that maintained relatively high hydrolysis yields. In this system, the silica particles on which the cellulase cocktails were immobilized likely acted analogous to scaffoldin, and thus created a cellulosome-like structure that led to synergistic enzyme hydrolysis of insoluble crystalline cellulose.
Immobilization of cellulases on silica to create a cellulosome like structure also opened up the possibility of reusability. Reusing enzymes by immobilizing them on a support is a promising way to make biorefining more economically attractive [13,14]. Much research has gone into developing enzyme immobilization techniques and measuring enzyme reusability on a variety of supports. However, unlike our process, most alternative immobilization techniques are complicated and involve expensive and/or toxic chemicals or supports [15-17]. Immobilization of cellulases on silica may enable reuse of enzymes in multiple cycles of hydrolysis, which could ultimately reduce capital and operational costs of biorefineries. An additional challenge for the applicability of immobilized cellulose in studies is the complex nature of the substrate used. The majority of studies used single, pure substrates such as microcrystalline cellulose, carboxymethyl cellulose (CMC), filter paper, Avicel, or xylan to evaluate the retained enzymatic activities of immobilize cellulases [19-21]. However, the activities of cellulose cocktails depend on the characteristics and availability of the substrate [22]. In addition, the compositions of industrial lignocellulosic biomasses are intricate; they are mixtures of crystalline cellulose, amorphous cellulose, hemicellulose, and lignin. Therefore, the hydrolytic behaviors of the immobilized cellulases on lignocellulosic biomass are presumably different from those on pure model substrates. Thus, it is critical to investigate the application of immobilized cellulases on complex lignocellulosic feedstocks. Additionally, it is also important to systematically understand how the various components of lignocellulose affect the activity of immobilized cellulases. Such analyses are rare in the current literature in the field of immobilized cellulases. In this study, two industrial lignocellulosic feedstocks were used: steamexploded poplar and waste office automation (OA) paper. Canada is one of the world’s largest forestry product producers and these growing industries produce a large amount of lignocellulosic biomass, such as sawdust and sustainably harvested wood from forestry industries, and paper sludge from paper industries [23]. Steam-exploded poplar was used as an example of wood-based biomass, and waste OA paper was selected as an example of paper-based biomass. The use of waste paper as a substrate in the field of cellulase immobilization studies is limited. We theorized that our immobilized cellulase systems, which create a cellulosome-like environment, would also promote efficient reusability. In addition, we hypothesized that if the immobilized cellulases can efficiently hydrolyze crystalline cellulose, it will also effectively degrade cellulose exhibiting amorphous structures as well as lignocellulosic biomass. This hypothesis was investigated through the detailed examination of the hydrolysis of two crude lignocellulosic biomass sources (steam-exploded poplar and waste OA paper) and three model substrates (commercial cellophane paper, phosphoric acid swollen cellulose (PASC), and xylan). This study is unique in that it systematically studied the hydrolysis of each lignocellulose component as model substrates as well as actual industrial lignocellulosic biomass.
Materials
Microcrystalline cellulose from Thermo Fisher Scientific Inc. (MA, USA) was used to assess reusability of immobilized cellulases. Another microcrystalline cellulose was purchased from Sigma-Aldrich Co. (MO, USA) and was used to prepare phosphoric acid swollen cellulose (PASC) as previously described [24]. Xylan from beechwood (poly(β- D-xylopryranose)) was also purchased from Sigma-Aldrich Co. Cellophane paper was purchased from Thermo Fisher Scientific Inc. and shredded by an office shredder to the size of 4 × 26 mm. Steam exploded poplar was kindly provided by Mascoma Canada Inc. (ON, Canada). According to the provider, it contains 42.1% cellulose, 25.4% hemicellulose, and 32.5% lignin. The pretreated poplar samples in wet conditions were stored at -20°C and thawed at room temperature prior to use. Our laboratory provided the waste office automation (OA) paper, which was originally purchased from OfficeMax Grand & Toy® (Premium copy paper, #99115, ON, Canada) and shredded by an office shredder to the size of 4 × 26 mm. OA paper can contain 55.7 to 87.4% cellulose, 4.7 to 13% hemicellulose, 0.93 to 5.78% lignin, and 1.4 to 25.47% other material [25]. The OA paper used in this study did not contain inorganic coatings and most likely has a composition similar to that described by Guerfali et al. [25]: 78.6% cellulose, 4.7% hemicellulose, 1.2% lignin, and 15.5% other material. Two commercial cellulase preparations were used in this study and were arbitrarily denoted in a previous work as Cellulases 1 (C1) and Cellulases 2 (C2) [18]. C1 was isolated from Trichoderma reesei ATCC 26921 and purchased from Sigma-Aldrich Co. C2 originated from Trichoderma reesei and Aspergillus niger and was kindly provided by NOVOZYMES North America Inc. (NY, USA). Two different types of silica were used as supports: Fumed silica S5130 (S1) from Sigma-Aldrich Co. and Davisil chromatographic silica 633N (S2) from Thermo Fisher Scientific Inc. S1 is a non-porous silica with a particle diameter of 7 nm and surface area of 390 ± 40 m2/g. S1 forms highly branched threedimensional molten matrices consisting of chains 10-30 units long. S2 is a porous silica with pore diameter of 60 Å, pore volume of 0.8 ml/g, particle diameter of 47-60 μm, and surface area of 500 m2/g, which remains as insoluble individual particles in solution. Different combinations of cellulases and silica were prepared in this study, and are described as follows: C1S1, C1S2, C2S1, and C2S2.
Determination of enzyme activity
The total cellulase activity was determined using the filter paper unit (FPU) assay [26]. To make the two cellulases comparable, the total cellulase activities were standardized at 2 FPU/ml. The activity of 1 FPU corresponded to 0.88 mg and 5 mg of C1 and C2, respectively. The same amounts of enzyme were employed in the immobilized and free cellulases for comparison purposes.
Cellulase immobilization
Immobilization was conducted under previously determined optimal conditions: C1S1, pH 5.0, 0.3 M ionic strength, 30 mg of support amount; C1S2, pH 5.0, 0.3 M ionic strength and 120 mg; C2S1, pH 5.0, 0.2 M ionic strength, and 30 mg; C2S2, pH 6.0, 0.2 M ionic strength, and 120 mg [18]. Immobilization was carried out as follows. C1 and C2 (2 FPU) were individually suspended in 1 ml of respective phosphate buffer, and then transferred to 1.5 ml microcentrifuge tubes containing S1 (30 mg) or S2 (120 mg). Samples, negative controls (silica with no cellulases), and positive controls (cellulases without silica) were individually mixed by pipetting and placed at 4°C for 2 h. The tubes were subsequently vortexed, centrifuged (8087 xg, 30 sec), and the pellets were then washed three times with 1.0 ml of respective phosphate buffers and finally suspended in 1.0 ml of 0.1 M phosphate buffer (pH 5.0). Based on a previous study, the loading efficiencies are expected to be between 90-95% (w/w) [18].
Hydrolysis
Hydrolysis of substrates (cellophane paper, PASC, xylan, steamexploded poplar, and waste OA paper) was examined after exposure to cellulases at 50°C for 24 h. The free cellulase solutions (C1 and C2) were prepared in 1 ml of 0.1 M phosphate buffer (pH 5.0). Immobilized (C1S1, C1S2, C2S1 and C2S2) and free (C1 and C2) cellulases were pre-incubated at 50°C in a water bath for 3-5 min prior to addition of 35 mg (dry weight) of individual substrate. Hydrolysis reactions were terminated after 24 h by placing the samples in a boiling water bath for 5 min followed by centrifugation at 8087 × g for 30 sec. The supernatant and solid fractions were stored at 4°C for further analysis. All reactions were performed in triplicate. The total reducing sugar concentration was measured using the DNS method [27]. Individual sugars in the hydrolysates were measured by high performance liquid chromatography (1200 series, Agilent Technologies, CA, USA) using an Aminex HPC-87H column (Bio-Rad Laboratories Inc., USA) at 60°C controlled by ChemStation software (LC system Rev. 04.01, SP1; Agilent Technologies). Sulfuric acid (5 mM) was used as the mobile phase at 0.6 ml/min at 60°C. A refractive index (RI) detector (G1362A, Agilent Technologies) was used for signal detection. External standards were used to calculate sugar concentration. Sugar content was expressed as hydrolysis yield [28], which was calculated using equation (1) as shown below:
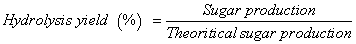
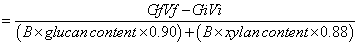 (1)
(1)
Where, Gi and Gf are the initial and final concentrations of sugar, respectively, Vi and Vf are the initial and final volumes of the enzyme mixture, respectively, and B is the dry weight of the biomass. The conversion factor for glucose to equivalent glucan is 0.90, while that of xylose to the equivalent xylan is 0.88. The glucan and xylan contents in microcrystalline cellulose, cellophane paper, and PASC are 1.0 and 0.0, respectively; the glucan and xylan content of steam-exploded poplar were 0.48 and 0.25, respectively (according to the manufacture); the glucan and xylan content of waste shredded paper were 0.80 and 0.10, respectively [25].
To compare our results with those from other studies, hydrolysis yields were also converted to retained enzyme activities using the following equation (2):
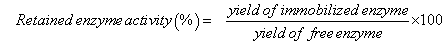 (2)
(2)
The concentrations of cellobiose and glucose as measured by HPLC were converted to carbon concentration using equations (3) and (4) below:
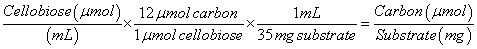 (3)
(3)
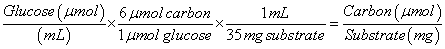 (4)
(4)
Recycling of immobilized cellulases
To assess the reusability of the immobilized enzymes, microcrystalline cellulose substrate (35 mg) was added to the enzyme/ silica mixtures and the hydrolysis yields (%) were determined after 24 h. The mixtures were then centrifuged (8087 xg, 30 sec) to separate the supernatants from the solid fractions. The supernatants were boiled for 5 min and stored at 4°C, while the solid fractions were washed three times with 1 ml 0.1 M phosphate buffer (pH 5.0) to remove any cellulose or reaction products. The solid fraction was then resuspended in 1 ml 0.1 M phosphate buffer (pH 5) prior to addition of fresh microcrystalline cellulose (35 mg) for the subsequent hydrolysis reaction. The hydrolysis and washing cycles were repeated a total of 9 times. Relative activity was calculated from hydrolysis yields using the following equation (5).
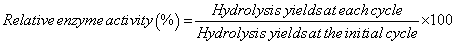 (5)
(5)
Statistical analyses
All experiments were performed in triplicate and statistical analyses were performed using TIBCO Software S+ 8.2 for Windows (TIBCO Spotfire, MA, USA).
Reusability of immobilized cellulases
In order to reduce the costs associated with enzymatic digestion of cellulosic material, we investigated whether immobilized cellulases could be reused for several successive rounds of hydrolysis, which would be extremely advantageous in an industrial setting. Our group previously examined the enzymatic activities of immobilized cellulases [18]. To assess the reusability of our immobilized enzyme systems, hydrolysis yields were examined during 9 successive 24 h hydrolysis reactions (Figure 1). Immobilized C1 possessed >40% of its enzymatic activity in the 6th cycle regardless of which immobilization silica was used. Conversely, C2 possessed only 23% and 29% of its activity in the 6th cycle when immobilized on S1 and S2, respectively. However, C2S1 possessed 54% of its enzyme activity in the 4th cycle and that of C2S2 was 50% in the 5th cycle. During the 4th cycle, C1S1 and C1S2 maintained 66% and 77% of their activity, respectively. Taken together, using our immobilization system, C1 and C2 maintained ≥ 50% of their activity for at least 4 cycles. It should be noted that cellulases immobilized on S1 had much higher initial hydrolysis yields, which corresponds with our previous studies and is likely due to the porous nature of S2 [18]. The cellulases likely get trapped in the pores of S2, which leads to higher levels of relative enzyme activity and better reusability. However, entrapment of the cellulases also limits substrate accessibility if the substrate cannot easily diffuse into the porous support, which can also lower levels of hydrolysis. Indeed, our previous studies showed that some microcrystalline cellulose is too large to enter the pores of S2 and thus cannot serve as a substrate for entrapped cellulases [18]. The relatively low rates of enzyme hydrolysis observed in all four systems were expected as we used microcrystalline cellulose for these experiments, which is composed of cellulose that is highly ordered and more resistant to hydrolysis. Nevertheless, we chose to use this substrate as crystalline cellulose is prevalent in lignocellulosic feedstocks that are receiving greater attention in industrial applications. Taken together, our results demonstrated that immobilized cellulases can be reused for successive hydrolysis reactions of 24 h while maintaining a significant amount of their enzymatic activity. There are other studies showing good reusability of immobilized cellulases. For example, Liang et al. achieved 83% of relative enzyme activity in the 5th cycle [15]; Alahakoon et al. realized 76% of the activity in the 10th cycle [16]; and Abraham et al. accomplished 70% of relative activity in the 3th cycle [17]. However, their immobilization procedures were more complex and required more expensive or toxic chemicals and supports than our method. In fact, Liang et al. covalently immobilized cellulase cocktails on a polymer synthesized with methacrylic acid, 2-(dimethylamino) ethyl metacrylate, and butyl methacrylate [15]. Alahakoon et al. synthesized aldehyde functionalized magnetic particles using 3-sminopropyltriethoxysilane, ammonium hydroxide, ethanol, and glutaraldehyde, and then covalently immobilized cellulases cocktail on the nanoparticles [16]. Moreover, Abraham et al. prepared cellulases using iron (III) chloride hexahydrate, iron (II) chloride tetrahydrate, and zinc chloride under 150°C for 12 h. The magnetic particles were then activated with glutaraldehyde while sonicating, followed by cellulose immobilization [17]. Compared to those procedures, our approach, which relied on a simple adsorption method using industrially feasible silica particles and phosphate buffer, is more environmentally friendly. Furthermore, the successful reuse of our immobilized cellulases may reduce the operational costs associated with the breakdown of cellulosic material in industrial processes. Future work will examine the possibility of maintaining 100% enzymatic activity each cycle by adding very small amounts of fresh cellulase to the used immobilized systems.
Hydrolysis of amorphous cellulose
To systematically study the efficacy of immobilized cellulases on individual components of lignocellulose, three model substrates were hydrolyzed: cellophane paper and PASC for amorphous cellulose regions, and xylan as a hemicellulose component. Hydrolysis of microcrystalline cellulose using our immobilized cellulase systems were examined previously [18]. To understand the activity of immobilized cellulases on amorphous cellulose, two model substrates, cellophane paper and PASC, were hydrolyzed. Since our previous research showed that immobilized cellulases could efficiently hydrolyze crystalline structures [18], we hypothesized that the immobilized cellulases would be efficient at hydrolyzing amorphous structures that are packed much more loosely and are therefore easily accessible. Indeed, the hydrolysis yields obtained when immobilized cellulases were exposed to cellophane paper and PASC, 52% to 73% (Table 1), were much higher than those obtained from hydrolysis of crystalline cellulose, which ranged from (14-25%) [18]. The retained enzyme activities of the immobilized cellulases were 60-78% of their respective unbound control enzymes. However, total sugar and glucose production was significantly lower than what was observed using the free cellulases (p<0.05; Figure 2). One likely explanation for this discrepancy is that although amorphous cellulose is more loosely packed, it is less accessible for the immobilized cellulases, which cannot diffuse throughout the mixture. Alternatively, immobilization may have modified the protein structure responsible for cleavage of the amorphous region. However, even though the retained enzyme activities were relatively lower in our immobilized cellulases compared to the corresponding free cellulases, the reusability of these immobilized cellulases may make them industrially feasible.
| Total hydrolysis yield (%) | |||||||
|---|---|---|---|---|---|---|---|
| C1S1 | C1S2 | C1 | C2S1 | C2S2 | C2 | ||
| Amorphous cellulose | Cellophane paper | 61 (± 7.1)a | 56 (± 1.5)a | 93 (± 0.60)b | 73 (± 2.2)A | 57 (± 1.4)B | 93 (± 1.2)C |
| PASC | 52 (± 1.3)a | 53 (± 0.34)a | 73 (± 1.1)b | 57 (± 0.48)A | 58 (± 0.61)A | 73 (± 3.7)B | |
| Hemicellulose | Xylan | 42 (± 1.2)a | 31 (± 0.71)a | 50 (± 2.7)b | 35 (± 4.4)A | 35 (± 0.68)A | 51 (± 3.2)B |
| Lignocellulosic biomass | Steam-exploded poplar | 35 (± 0.59)a | 23 (± 0.12)Bb | 56 (± 1.1)c | 45 (± 3.0)A | 42 (± 1.1)A | 73 (± 1.0)B |
| Waste OA paper | 12 (± 1.7)a | 16 (± 0.33)a | 36 (± 0.44)b | 22 (0.82)A | 18 (± 0.41)A | 45 (± 0.53)B | |
Table 1: Total hydrolysis yields obtained using various substrates treated with free enzymes (C1 or C2) or immobilized cellulases (C1S1, C1S2, C2S1, C2S2). Hydrolysis reactions were conducted in 0.1 M phosphate buffer (pH 5.0) at 50°C for 24 h using a water bath; n = 3; Mean ± Standard Error. For each substrate, values with the same subscript letter are statistically similar while values with different subscript letters are statistically different (p<0.05). For the subscript letters, lower case letters are used for all systems (free or immobilized) incorporating C1, whereas upper case letters are used for C2. PASC: Phosphoric acid swollen cellulose; OA: Office automation.
Figure 2: Carbon production observed through hydrolysis of amorphous cellulose (35 mg/mL). a) Hydrolysis of cellophane paper using free cellulase (C1) and immobilized cellulases (C1S1, and C1S2); b) As described in a), but using C2, C2S1 and C2S2; c) Hydrolysis of phosphoric acid swollen cellulose (PASC) using free cellulase (C1) and immobilized cellulases (C1S1 and C1S2); d) As described in c), but using C2, C2S1 and C2S2. Hydrolysis reactions were conducted in 0.1 M phosphate buffer (pH 5.0) at 50°C for 24 h using a water bath; n = 3; Mean ± Standard Error. Values that were significantly higher or lower (p<0.05) than those obtained using the corresponding free cellulase are denoted with an asterisk (*) and double asterisk (**), respectively.
Also, the hydrolytic properties of the cellulases might be improved if the immobilization system or the reaction conditions are modified and optimized for amorphous cellulose. Reaction conditions used in the current systems were chosen based on hydrolysis yields obtained using microcrystalline cellulose [18]. The amount of cellobiose produced by the free and immobilized C1 systems were statistically similar regardless of if cellophane paper or PASC was used, ranging from 6.3 to 8.6 μmol/mg substrate (Figure 2). Conversely, almost no cellobiose was generated by free C2. C2 is a mixture of cellulases from both Tricoderma ressei and Aspergillus niger, the latter of which is known to produce large amounts of β-glucosidase, the enzyme responsible for the hydrolysis of cellobiose to glucose [29]. Conversely, C1 is derived from Tricoderma reesei only, and thus may only contain small amounts of β-glucosidase. This may explain the difference in cellobiose observed using free C1 and C2. Interestingly, significant cellobiose was observed in all immobilized systems using C2. It is possible that the immobilization processes disrupted the β-glucosidases present in C2, or these enzymes might not be efficiently adsorbed on the silica support [18]. Further experiments to determine the reasons behind the reduced cellobiose conversion are necessary.
Hydrolysis of hemicellulose
Steam-exploded poplar contains approximately 25.4% hemicellulose according to the manufacture, while waste OA paper contains 4.7% [25]. It is therefore important to examine if C1 and C2 retain their hemicellulase activities in addition to their other lignocellulose-decomposing enzyme activities, and if the immobilized cellulases can efficiently hydrolyze hemicellulose. To this end, the immobilized and free cellulases were analyzed for their ability to digest xylan, a typical component of hemicellulose. The observed hydrolysis yields ranged from 31-51% (Table 1), which demonstrates that C1 and C2 possess substantial hemicellulose activities. The total hydrolysis yields of the immobilized cellulases using xylan as a substrate were statistically lower than those of the free cellulases (p<0.05; Table 1). In addition, other than C1S1, the immobilized cellulases did not produce xylose and xylobiose as efficiently as the free cellulases (Figure 3). The total sugar production was slightly lower in C1S1 than that in free C1 enzyme (p<0.05), but the amounts of individual sugars were not statistically different (p>0.05). This result is promising and may imply an application for C1S1 in the breakdown of lignocellulosic biomass containing high hemicellulose fractions, such as willow, wheat straw, and rice straw [30]. Other immobilized cellulose systems possess hemicellulases activities, but did not hydrolyze xylan as efficiently as free cellulases. The reason for the lower hydrolysis yields for most of the immobilized cellulases compared to the corresponding free cellulases was possibly because the immobilization process negatively affects the hemicellulases, resulting in a decrease in hemicellulase activity. Alternatively, this observation may result from the amorphous nature of the hemicellulose, which renders it more accessible to free hydrolytic enzymes as was discussed in 3.2.
Figure 3: Carbon production observed through hydrolysis of a common hemicellulose component, xylan (35 mg/mL). a) Xylan hydrolysis using C1, C1S1, and C1S2; b) Xylan hydrolysis using C2, C2S1, and C2S2. Hydrolysis reactions were conducted in 0.1 M phosphate buffer (pH 5.0) at 50°C for 24 h using a water bath; n = 3; Mean ± Standard Error. Values that were significantly lower (p<0.05) than those obtained using the corresponding free cellulase are denoted with a double asterisk (**).
The retained enzyme activities of up to 62% to 84% were higher than those previously observed in the literature. For example, Xu et al. immobilized cellulases cocktail on a polymer, Eudragit L-100, and they achieved 59% of retained enzyme activity compared to the free cellulases [31]. Xu et al. used magnetic nanoparticles as a support for the immobilization and hydrolysis of xylan; the retained enzymatic activity of the immobilized cellulases was 59% [32]. Moreover, Mandali and Dalay immobilized cellulases on porous Siran™ glass microcarriers and observed 8-19% retained enzyme activities [33]. Therefore, the immobilized cellulases described here may be suitable for the hydrolysis of the hemicellulose fraction in lignocellulosic biomass. To make our immobilized systems more industrially feasible, the different immobilized cellulase systems described in this report can be combined together to achieve maximal lignocellulosic hydrolysis.
Hydrolysis of industrial lignocellulosic biomass
Finally, the effects of immobilization on the hydrolysis of lignocellulosic biomass, steam-exploded poplar and shredded waste OA paper, were examined (Figure 4). The steam-exploded poplar was used as an example of wood-based lignocellulosic biomass and contained 42.1% cellulose, 25.4% hemicellulose, and 32.5% lignin. The total hydrolysis yields of the immobilized cellulases were statistically lower than those of free cellulases (Table 1). However, the immobilized cellulases still retained relatively high enzymatic activities. The retained enzyme activities of C1S1, C1S2, C2S1, and C2S2 in the steam-exploded poplar hydrolysis relative to the free cellulases were 62%, 41%, 62%, and 58%, respectively, which are higher than most of the other reported systems that employed various lignocellulosic biomasses. For instance, Sutarlie and Yang hydrolyzed palm oil fiber using hybrid cellulase aggregates with silica gel and achieved a 28% retained enzyme activity [34]. Xu et al. immobilized a cellulase cocktail on magnetic nanoparticles and applied them to steam-explode corn stover [32]. This group obtained 33% retained enzymatic activity. Additionally, Mandali and Dalay immobilized cellulases and hemicellulases on porous glass beads and hydrolyzed corn stover [33]. The authors achieved 7-14% retained enzyme activities. Thus, the immobilized cellulases described in this report may be successfully employed to hydrolyze lignocellulosic biomass. Figures 4a and 4b show the sugar compositions of steam-exploded poplar hydrolysates. The amount of glucose and cellobiose produced in immobilized systems using C1 was statistically lower than those obtained by their corresponding free cellulases in steam-exploded poplar hydrolysis (p<0.05). A similar reduction in glucose was observed using immobilized C1 systems and amorphous cellulose substrates (Figures 2a and 2c). However, when using steam-exploded poplar, a decrease in cellobiose production was also observed. One explanation for the drop in glucose and cellobiose yields in the hydrolysates of steam-exploded poplar may result from cellulose fractions being embedded in lignin and pectin fractions. Thus, the immobilized cellulases might have difficulty accessing the cellulose fibers.
Figure 4: Carbon production observed through hydrolysis of lignocellulose biomass (35 mg/mL). a) Hydrolysis of steam-exploded poplar using C1, C1S1, and C1S2; b) As described in a), but using C2, C2S1, and C2S2; c) Hydrolysis of waste office automation (OA) paper using C1, C1S1, and C1S2; d) As described in c), but using C2, C2S1, and C2S2. Hydrolysis reactions were conducted in 0.1 M phosphate buffer (pH 5.0) at 50°C for 24 h using a water bath; n = 3; Mean ± Standard Error. Values that were significantly higher or lower (p<0.05) than those obtained using the corresponding free cellulase are denoted with an asterisk (*) and double asterisk (**), respectively.
For the immobilized C2 cellulases, the amounts of glucose produced were also lower relative to the free C2 cellulases, but more cellobiose was generated. A similar result was observed using amorphous cellulose substrates (Figure 2) and as mentioned previously, likely stems from differences in β-glucosidase contents. Xylose production did not significantly differ between all immobilized cellulases and their respective free enzyme reactions (p<0.05; Figure 4), which was different from the results of xylose hydrolysis (Section 3.3). This is presumably due to the fact that hemicellulose accounts for only 25% of the steamexploded wood. Thus, the immobilized cellulases did not likely have difficulty hydrolyzing the small amount of hemicellulose present in this substrate. Waste OA paper was used as an example of industrial waste paper sludge biomass, which is novel in the field of immobilized cellulases (Figure 4c and 4d). This material is thought to contain 78.6% cellulose, 4.7% hemicellulose, 1.2% lignin, and 15.5% other material [25]. The total hydrolysis yields of free C1 and C2 cellulases were 36% and 45%, respectively, which are comparable to values obtained in the literature [35]. The retained enzyme activities of C1S1, C1S2, C2S1, and C2S2 in the waste OA paper hydrolysis compared to the corresponding free cellulases were 34%, 43%, 48%, and 41%, respectively (Table 1). The hydrolysis yield values obtained using the immobilized cellulases and OA paper were the lowest of all the substrates examined (Table 1). This may result from the size of the paper strips, which were the largest substrates applied in this report (4 × 26 mm). Therefore, the immobilized cellulases may have had great difficulty accessing the paper fibers. Actual paper sludge consists of liquid suspensions containing dispersed paper fibers. Thus, the substrate accessibility of the immobilized cellulases to the fibers might increase when paper sludge is used rather than OA paper. However, it should also be noted that paper contains processing chemicals that may negatively affect the immobilized cellulases. All sugars in the hydrolysates obtained from the immobilized cellulase systems, except cellobiose in C2S1 and C2S2, were statistically lower than values observed in hydrolysates recovered from treatment with their corresponding free enzymes. This was expected since the total sugar production was also significantly lower (p<0.05) for all immobilized systems, relative to controls, and is likely a result of the large size of the paper strips as discussed previously. The cellobiose productions for C2 immobilized cellulases were higher than those for free C2, which corresponds with the results of steamexploded poplar hydrolysis; again, β-glucosidase activities were presumably lost during the immobilization process. It should be noted that the immobilization and reaction conditions used in this report were previously optimized for microcrystalline cellulose hydrolysis. Thus, the optimization of conditions for specific target lignocellulosic biomasses might greatly improve hydrolysis yields. Further research is required.
Two different commercial cellulases, termed Cellulases 1 (C1) and Cellulases 2 (C2), were separately immobilized on non-porous silica (S1) and porous silica (S2) using our easy, efficient, safe, and cheap methodology. All immobilized cellulases could be reused at least 4 times while maintaining 50%-77% of their activity, relative to the first cycle. Thus, our immobilization process using silica not only allows for efficient hydrolysis through creation of a cellulosome-like structure, but it also promote enzyme recycling, which makes it an extremely promising method from an industrial point of view. Systems incorporating C1 seemed to be more successful, maintaining >40% of its activity in the 6th cycle of use when immobilized on either S1 or S2. Compared to the results from other immobilization systems in the literature, our immobilized cellulases maintained higher retained enzyme activities using a wide range of substrates: amorphous cellulose, hemicellulose, and lignocellulosic biomass. The successful hydrolysis of such substrates strongly suggests that our immobilization method can be applied to a wide range of enzymes including a variety of cellulases and xylanases. Finally, although cellulase immobilization studies typically employ only model substrates, our research incorporated two lignocellulosic biomass substrates: steam-exploded poplar and waste OA paper. Although hydrolysis yields obtained using these substrates were lower than those observed when using model substrates, these experiments demonstrated successful application of our immobilized cellulases to breakdown lignocellulosic feedstocks that are of great interest industrially. Future studies will be aimed at increasing hydrolysis yields though optimization of enzyme components.
This study was funded by Alberta Enterprise and Advanced Education (formerly Alberta Advanced Education and Technology), Alberta Agricultural Research Institute, Alberta Innovates Bio Solutions, Alberta Ingenuity GraduateScholarship in Nanotechnology from Alberta Innovates Technology Future, Alexander Graham Bell Canadian Graduate Scholarship from Natural Sciences and Engineering Research Council of Canada (NSERC), and the Biorefining Conversions Network (BCN).