Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Review - (2024)Volume 17, Issue 3
Gene editing is a technique of improving the genetic makeup of organisms by deletion of infected alleles, the wild type of sequence modified or integration of exogenous Deoxyribonucleic Acid (DNA) to obtain new gene function. Gene editing is achieved by zinc finger nucleases, transcriptional activating like effector nucleases and recently with newly modified genetic tools known as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas9) is used to edit the non-functional genes into functional genes. The natural defense mechanisms of prokaryotes against phage invasion coordinated in three stages, adaptation, CRISPR Ribonucleic Acid (crRNA) synthesis and targeted interference. The mechanisms of CRISPR/Cas9 actions completed three parts that are recognition, cleavage and repairing process. The engineered Guide RNA (gRNA) components derived from the natural crRNA, the guiding part and loop forming Trans-Activating CRISPR RNA (tracrRNA) (scaffold) artificially combined to engineered Single Guide RNA (sgRNA). The nuclease and recognition sites make Cas9 protein. Currently scientific communities focused on CRISPR/Cas9 based gene editing due to its high effectiveness than other gene editing tools. Since recent times, gene editing tools were widely applicable in plants for yield increment, disease resistant also drought resistant and in human diseases like cancers, haemophilia and cystic fibrosis. The gene editing technology is also becoming widely applied for the targeted treatment of animal diseases such as porcine reproductive and respiratory syndrome, Porcine Epidemic Diarrhoea (PED) and transmissible gastroenteritis, bovine tuberculosis and mastitis. Therefore, this review was aimed to understand the recent updates on genome editing technology and its role for the targeted animal diseases.
Gene editing tools; CRISPR/Cas9; Applications; Targeted animal diseases
In the 21st century gene editing technology is the main concern and globally acceptable technique that highly revolutionized the production of agriculture, environment protection, control and prevention of human and livestock diseases and other type of disease aiming to improve global food security and reduce food waste throughout the production chain [1]. Gene editing is a new modern engineering applied mainly for curing health abnormalities even minimizing the expense of medication where users enables to make alterations of DNA sequence of an organism’s genetic make-up either in in vivo or ex vivo [2,3]. Alteration of DNA or gene editing can be done by disruption, restoration, insertion or deletion systems. The development of platforms for gene editing simplifies the understanding of disease pathogenesis, autoimmune and inflammatory response using either cell systems or animal models in order to study diseases of monogenic disorders (disease caused by single genetic defect) like cystic fibrosis, haemophilia, sickle cell anaemia and cancer [4].
As the patient genomes become sequenced, vast number of mutations associated with various diseases becomes clearly determined and identified. Genome editing manipulates specific gene loci in order to gain genome modifications in the form of insertions, deletions or point mutations essential for identification of functional targeted genes and regulatory factors [5,6]. Designer nucleases like Dimeric-type IIS Restriction Enzyme (FokI) and Cas9 are used as gene editors by cleaving DNA strands of the entire nucleus cells in a specific cleavage site both in natural and engineered forms. DNA repairing considered as introducing a precise genetic change at a target locus of targeted region of gene interest. Zinc Finger Nuclease (ZFN) and Transcription Activator like Effector Nuclease (TALEN) are classified under first category class genome edition technology tools. Both tools were modular proteins containing an adaptable DNA binding domain fused to the nuclease domain of FokI proteins. In ZFNs each zinc finger binds to three DNA bases whereas each TALEN repeat binds a single base. ZFNs and TALENs were employed as pairs which recognize opposing DNA strands and orient their fused FokI monomers to bring together on the intervening sequence to form active enzyme dimer that cleaves both strands [7,8].
The second category class gene editing tool is the CRISPR which is associated with Cas enzyme gene, CRISPR/Cas presently used as the most popular designer nucleases cleaving non-specific target sites to generate desired or error alteration sequences [9]. CRISPR was discovered from innate immune mechanisms of various prokaryotes defending the invading viruses and nucleic acids performing during their own DNA cleavage roles [8]. Gene editing treatment made for patients is effective for extended period in individual life time. Fatal diseases of the 21st century like cancer, autoimmune diseases and allergies caused by individual abnormal immune responses in hosts. As advancement of human knowledge, gene editing is becoming the first options to be developed for combating global pandemic and genetic diseases such as degenerative nerve cells, weak immune status, metabolic disorders and various forms of cancers are some of considerable in treatment trials where latest successful achievements obtained by introducing the corrected gene into the cells using various techniques of gene delivery tools.
Gene editing technology
Gene editing technology is the driver of sequence specific alteration of genetic material by precisely modified insertion, deletion, repair of the defective gene or replacement of gene responsible for diseases at a specific site along with genomic sequence or in patient’s cells by knocking out unnecessary traits. Repaired traits introgressed into genomic sequence of a patient by molecular gene editing tools that take an advantage of site directed DNA repair after strand breakage primarily by engineered endonucleases. At the targeted sites of DNA sequence were Double-Strand Breaks (DSB) formed. The role of DSB at the desired DNA sequence or genomic loci was to activate two competing branching DNA repair systems, the Non-Homologous End-Joining (NHEJ) or Homology-Directed Repair (HDR) [10]. The oligonucleotide templates used for building single nucleotide changes in the targeted genome that allows directly transforming wild and novel DNA sequence variants into individuals and stimulating HDR to eliminate the need for transgene-dependent selection [11]. The similarities and differences of the three programmable nucleases were discussed in Table 1.
| Properties | ZFN | TALEN | CRISPR/Cas9 |
|---|---|---|---|
| Origins | More in eukaryote | Bacteria (Xanthomonas) | Bacteria (S. pyogenes) |
| Specificity | ZFP | TALE | sgRNA (crRNA + tracrRNA) |
| Cleavage mechanism | DSB induced by FokI | DSB induced by FokI | DSB or SSB induced by Cas9 |
| Target length | 18-36 bp (3 nt per ZF) | 30-40 bp (1 nt TALE binding sites start in T) | 20-22 bp + G-rich PAM (NGG) sequence (Cas9 binding sites) |
| Mechanisms of target | DNA-protein | DNA-protein | DNA-RNA |
| DNA recognition | Interaction, cut and repair | interaction, cut and repair | interaction, cut and repair |
| Fusions | Protein | Protein | Protein and RNA |
| Ease of design | Difficult | Moderate | Easy |
| Protein size | Small | small | big |
| Multi target KO | No | Difficult | Possible |
| Total of proteins | 2 | 2 | 1 |
Note: ZFN: Zinc finger nuclease; ZFP: Zinc Finger Protein; TALEN: Transcription activator like effector nuclease; TALE: Transcription activator-like effectors; CRISPR/Cas9: Clustered regularly interspaced short palindromic repeats; FokI: Dimeric-type IIS restriction enzyme; bp: Base pair; nt: Nucleotide targeter; DNA: Deoxyribonucleic acid; RNA: Ribonucleic acid; sgRNA: Single guide RNA; crRNA: CRISPR RNA; tracrRNA: Trans-activating CRISPR RNA; DSB: Double-strand breaks; SSB: Single-strand breaks; PAM: Protospacer-adjacent motif; T: Thymine; G: Guanine.
Table 1: Similarity and difference between commonly known gene editing tools.
Tools used for gene editing
Based on natural gene editing systems, synthetic gene editing nucleases such as homing endonuclease, ZFN, TALENs and at the latest time CRISPR techniques were developed due to single traits of diseases as well as the emerged pandemic diseases [12].
Homing endonucleases (meganucleases): Meganucleases is a low frequency cutters having a recognition sequence of 20-30 base pair (bp) in yeast. Homing is the process of gene conversion presenting in the three life domains. Restriction Enzyme (HE) is a DNA recognizing and cleaving enzyme known with very rare recognition sites in a portions of long target DNA or homing sites encoded by Open Reading Frame (ORF) in eukaryotes and prokaryotes genome. In HE the ORF were attached into homing sites to transfer into a sequence that lacks homologous alleles of introns and inteins. The mobile intervening sequences, (group I intron and inteins) together by ORF were transferred into the spliced target allele then repaired with DSB [13].
Introns are a segment of DNA sequences with variable length within a gene but not part of gene expression, spliced during Ribonucleic Acid (RNA) transcript. There are two groups of self-splicing introns. In group I, introns self-splicing occurred due to the presence of guanosine acid as a cofactor existed as essential genes. Group II introns self-splicing reaction was initiated by adenine forming a lasso assembly in Ribosomal RNA (rRNA), Transfer RNA (tRNA) and protein coding genes [13]. Four conserved protein motifs presented in meganuclease.
LAGLIDADG homing endonucleases (LAGLIDADG) family: A populous protein family, exist in all biological life domain encoded by ORF of mobile self-splicing introns [14]. LAGLIDADG recognizes at 13-40 bp intervals for cleavage. The homing sites of the DNA produce 3’ cohesive ends with 4 bp overhangs encoded in group II introns. HE serve as a dimer, which contains only one catalytic domain, and as a monomer consists of two catalytic domains. Recognition and excision of homing sites mediated by Homing Endonuclease (I-CreI) contains a 22 bp length altering introns and inteins into intronless alleles. The palindromic and non-palindromic sequence reads rely on the scaffolds of homo-monomer and homo-dimer. LAGLIDADG sequence motifs used for protein folding, catalytic activity and enable to classify meganuclease into I-CreI and I-CeuI families based on the presence of motifs number. Intron Encoded Endonuclease (I-DmoI) and Monomeric Homing Endonuclease (PI-SceI) families contain two motif sequences which act as monomers [2].
GIY-YIG homing endonucleases (GIY-YIG) family: GIY and YIG are the two short motifs found at the N-terminus, in the middle next to arginine and glutamine residue at C-terminus. This nuclease only found in group I intron. HE can recognize and cleaves only the intact DNA target not their mobile DNA. Homing Endonuclease I-TevI (I-TevI) is involved as DNA binding domains occurs in a freely standing ORF. The GIY-YIG sequence motifs occurred in a poorly conserved form with invariant residues. I-TevI was encoded in a mobile intron and interacts with its target DNA as a monomer. inteins necessarily encodes the ORF that was ready to insert into a protein coding sequence. The flexible linker in I-TevI joins the two catalytic domain and binding domains. Catalytic domain has cleavage site on both strands. The binding domain have groove for intron insertion site at the two strands [15].
His-Cys box homing endonucleases (His-Cys) family: In this protein, family, histidine and cysteine residues found in a conserved motif at N-terminus encoded into group I intron to alter the mobile intron into intronless alleles of their host genes [16]. Intron-Encoded Endonuclease (I-PpoI) is a small protein that binds its homing site as a homodimer to induce the DNA to have a curve shape and catalyses a DSB across the target DNA minor groove to 3′ ends. The dimers can make a curve form in the DNA closely to cleavage site in order to opens the minor groove for DSB. Moulds, algae, fungi and amoebae can utilize this type of protein family during their life cycles. In yeast, ORF confined into group I intron in a nuclear ribosomal DNA [17].
HNH endonuclease (HNH) family: This protein family have a double histidine and a single asparagine flanking the conserved motifs found as ‘ββα’, which consists a diverse nuclease, related proteins. H-N-H Homing Endonucleases (HNH) motif actually existed in colicins, transposases, restriction endonucleases, DNA packaging factors, group I and group II intron maturases [18]. HNH subfamilies are embedded into ORF to determine the structure and function of protein domains, reverse transcriptase, DNA repair enzyme and in various DNA-binding motifs [19].
I-HmuI Homing Endonucleases (I-HmuI) recognizes longer asymmetric DNA sites around 24 bp found close to the N-terminus. The HE of I-HmuI binds its target DNA as a monomer using two successive α helices and helix-turn-helix motif to link into a DNA. The structures of I-HmuI consists an active site of double β-strands and α-helix used to interacts with phosphate backbone at the minor groove closely to DNA cleavage site. The HNH cleavage activities depends on their catalytic diversity digested the Double-Stranded DNA (dsDNA) targets induced by two options either by DSB or single-strand nicks in the duplex DNA targets [18].
ZFN: Zinc is trace metal elements that have ability to appropriate regular functions in large number of proteins similarly in enzymes. In primary, secondary and tertiary structure of proteins their entire shape maintained by the presence of zinc like α and β sheet. For the first time a zinc cluster protein studied was the Gal4 Transcription Activation Factor (Gal4p) contributed as a transcriptional activator of genes carried out in the catabolism of galactose [20]. ZFN was protein guided genome editing tool synthetically modified in the form of hybrid protein serially arranged a ZFN domain that originated from naturally occurring prokaryote. ZFNs existed as fusions of non-specific DNA cleavage domain from the FokI restriction endonuclease combined with Zinc Finger Protein (ZFP), which used for its ability to make precise genomic modifications by inactivating defective gene and transcription factor [21]. FokI is an endonuclease enzyme found between DNA recognition domain and a catalytic domain, which is, grouped under type IIs restriction enzymes [22]. The name was derived from Flavobacterium okeanokoites after FokI genes was sequenced. It was used to recognize in irregular sequence and cleaves the double strand DNA outside of the recognition region. The two DNA binding domain recognizes a unique hexamer (6 bp) sequence of DNA. ZFP formed from two finger modules stitched together. A DNA-cleaving domain is the nuclease domain of Fokl. The DNA binding and DNA-cleaving domains after fused together a highly specific pair of genomic scissors is formed as it was depicted in Figure 1 [23].

Figure 1: Designer DNA-binding domains and genomic scissors. Note: DNA: Deoxyribonucleic Acid; FokI: Dimeric-type IIS restriction enzyme; A: Adenine; T: Thymine; C: Cytosine; G: Guanine.
ZFNs structure and mechanism of actions: In eukaryotes zinc finger domain of Cysteine2-Histidine2 (C2H2) has a hundred of ZFPs found to serve for DNA-binding motif. This domain entirely contains multiple cysteine and histidine residues, which are main ligands for zinc ion in proteins in order to stabilize their own folds. DNA binding activity of Zinc Finger (ZF) domains has an advantage in recognizing the desired DNA sequence while the DNA nuclease cuts the DNA of a gene to provide useful information such as gene regulation and gene editing [21]. ZFP domains are existed as conserved amino acids of Cysteine and Histidine (C2H2, C4 and C6) [20]. Every ZFP made from 30 amino acids with a DNA interaction of single α-helix motif stabilized by zinc ligation of two β-strands. ZFNs was engineered to recognize 3-4 bp sequences and 3 to 4 ZFPs re-joined in tandem to target specific genome sequences of 9-18 bp [24]. ZFPs are most abundant DNA binding motifs in eukaryotic genome able to recognize the 64 possible nucleotide triplets [25].
ZFN editing done by fusing a transcription factors found in eukaryotic proteins responsible for alter cell types and with the cleavage domain of Folk restriction enzyme. The FokI enzyme binds to the DNA recognition site to activate its cleavage domain site and remove the part of DNA strand. The two active ZFN monomers bind to complementary adjacent regions of DNA separated by the proper spacing to enable the formation of FokI endonuclease dimer creates site specific DSB in the target DNA of a sequence. Zebra fish and murine animal models were generated by ZFNs directly by injecting into their zygote [25,26]. Therapeutic applications of ZFNs in C-C Chemokine Receptor Type 5 (CCR5) gene were expressed in human Cluster of Differentiation 34 (CD34+) hematopoietic stem and progenitor cells was disrupted using NHEJ. Similarly, genetically modified cells provide a permanent supply of Human Immunodeficiency Virus (HIV) resistant cells by preserving the immune cells to cures HIV infection [27].
TALENs: TALEN is one of gene-editing strategy containing an artificial restriction enzyme in order to cleave site-specific sequence of DNA. As a TALENs compared with ZFNs, TALENs more preferred because of its design and lower cost [28]. The engineered Transcription Activator-Like Effectors (TALEs) fused at the desired DNA segment having the ability to display an array of TALE subunits. The non-specific endonuclease protein, FokI used for specific sequences to recognize the target genomic sites [29].
TALENs structure and mechanism of actions: The genomic DNA of DSBs repaired via HDR or NHEJ attained with a necessary editing of genomic information such as gene deletion, gene insertion and gene correction [30]. TALEN DNA binding domains can have 33-35 amino acids repeats while each of them recognizes single base pairs. TALENs can have around 20 active repeating units enable to limit the vectors capacity to deliver into target cells [31]. TALENs editing can be done by fusing the proteins of FokI cleavage domain with the bacterial binding domain of a TALE effector protein. The binding domain binds to a specific DNA sequence cleaved by the pair of FokI nuclease domain. The presence of long DNA recognition sequences within the TALE DNA binding domains makes the TALENs to be targeted is the custom selected loci precisely with minimal off-target effects or cytotoxicity [32]. The structure of TALEN is composed from a TALE with central repeat domain used for DNA binding, a truncated N-terminal Segment (NTS), a truncated C-terminal Segment (CTS) and a non-specific DNA cleavage domain from a Type IIS restriction enzyme called FokI. The ideal spacer length depends on the TALE scaffold construction. FokI nuclease domains dimerize the DNA cleavage activity. The classical TALEN tool desires two compartments of TALENs in order to bind with existing of two properly matching DNA target sites flanking an unspecific central spacer. A polypeptide is linker that connects two FokI nuclease domains [33].
CRISPRs: Naturally humans and animals internal body have their own first and second line defence of immune system for pathogens invading. In the natural host of bacteria adaptive CRISPR defence mechanisms from invading viruses passes 3 stages.
Adaptation: The invading phage viral DNA is processed into short DNA segments that incorporate into a bacterial new spacer (CRISPR sequence) between the repeats. The new bacterial RNA sequences copied from viral DNA used to memorize past infection based on a unique Protospacer-Adjacent Motif (PAM) phage and degrade the active site of phage that matches the spacer sequence of bacteria to protect from a new viral attack.
CRISPR RNA production: Palindromic segments the Cas genes and spacers in the host DNA undergo transcription together with Cas9. In transcription, bacterial DNA requires a single chain RNA. The RNA sequence is known as CRISPR RNAs (crRNA).
Targeting: The crRNAs guide the whole bacterial machinery using Cas9 memorize and damaged the incoming phage DNA once after earlier infection [34].
The structures and components of CRISPR/ Cas9:The genomic sequence of CRISPR comprises; CRISPR-related genes (Cas9 nuclease), non-coding RNA (crRNA) and a unique array of positive repetitive elements parts transcribed into Precursor-CRISPR RNA (pre-crRNA) as indicated in Figure 2 [35].
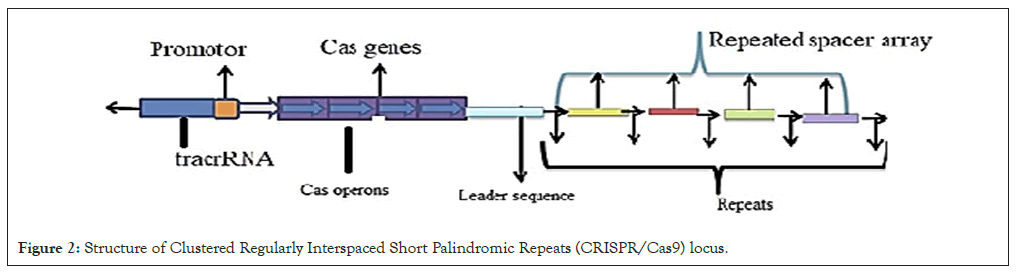
Figure 2: Structure of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas9) locus.
Guide RNA (gRNA) is constructed from scaffold sequence suited for Cas9 nuclease binding and a spacersequence having a length of 20 nucleotides responsible to cleave the double-stranded DNA in the presence of PAM [36,37].
The length of CRISPR sequence is nearly 21-48 b is a unique short sequence palindrome repeats separated by one another. Every short repetitive sequence is identified by aligning in line with an exogenous DNA target, a protospacer. The protospacer used for recognition of the target locus binding site for Cas9 using by its own signal. Cas9 have nuclease (NUC) and recognition site in its surface [38]. The target DNA of each typical spacer region located to adjacent PAM [39,40].
Mechanism of gene editing in CRISPR/Cas9:Mechanism of gene editing entails three parts.
1. Recognition was a key to the action of cleavage. REC domain plays a vital role during the interactions between sgRNA and Cas9 [6]. After a complex process sgRNA and Cas9 nuclease combined together to recognizes and binds at the target sequence. PAM is a short conserved DNA sequence useful for locating sgRNA- Cas9 binding to the target gene downstream to the cleaved site. The base pairing reactions reads and captures the interest DNA to protect unexpected self-mutilation and flanks cDNA to the seed sequence of sgRNA to produce a sgRNA-target DNA heterodupex and finally trigger R-loop formation [41]. The unwinding target DNA also relies on PAM [6].
2. Cleavage; RNA-DNA heteroduplex bonded together to form a dsDNA to stabilizes the PAM motif [42]. The HNH and Crossover Junction Endodeoxyribonuclease RuvC (RuvC) are the functional nickase domains activated by Cas9 guided with duplex RNA. The HNH and RuvC domains are one of the nuclease lobe located in Cas9 protein used to cut a site of single strand of target DNA in respective to one another. During cleavage, Cas9 cut the target DNA with HNH nuclease domain nicking the DNA sequence strand complementary to the gRNA. The RuvC domain cut the displaced strand to yield a site-specific DSB upstream to the PAM sequence. Helicase opens the double strands DNA [9].
Single-stranded cleavage is prone to mutations due to the presence of HNH and RuvC [43]. Engineered gRNA organized in two forms (crRNA, tracrRNA and Cas9) and (crRNA and Cas9) [44]. The target site was identified by the process of probing a PAM sequence and the interactions of matching gRNA with target DNA. In the presence of mismatched, the Cas9 immediately dissociated from the DNA. Cas9 triggers the DSB after the complementarity between sgRNA and target DNA have been adjusted themselves then generate energy enzyme enables to break dsDNA as illustrated (Figure 3) [45,46].
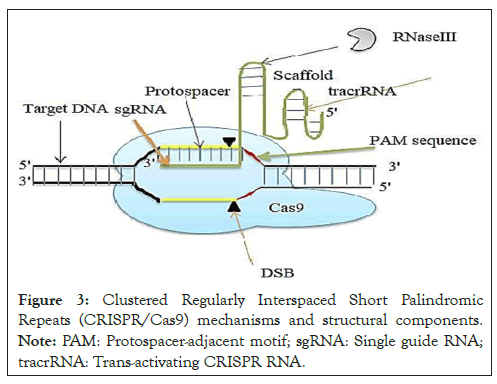
Figure 3: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas9) mechanisms and structural components. Note: PAM: Protospacer-adjacent motif; sgRNA: Single guide RNA; tracrRNA: Trans-activating CRISPR RNA.
The Cas1 and Cas2 endonucleases recognize the viral genome to break DNA into small fragments and insert them into bacterial genome as repeat-spacer units. The second phase is the immunity phase a subsequent viral invasion of bacteria to produce pre-crRNA based on recalling previously captured repeat-spacer units. The pre-crRNA fused with Cas9 endonuclease and the tracrRNA together to forms the crRNA-Cas9-tracrRNA complex [47-50].
Repairing systems, CRISPR/Cas9 generated DSB. DSB repaired by the instructions of NHEJ and HDR [48]. In NHEJ, the two freely separated ends of DNA fragments are re-joined together by enzymatic error prone mechanism by introducing random deletions or insertions of nucleotides. Thus, this mediation creates mutations at the desired target sites by the disruption of mutated/defective gene [51-55]. Indel mutations occurred within the coding region of the gene, the frame shift mutation and premature stop codon produced due to gene disruption or knockout. HDR mediated DNA repair requires specific exogenous DNA derived from dsDNA, sister chromatid, chromosome and Single-Stranded DNA (ssDNA) oligonucleotide as a repair template encoding the broken area to edit with homologous sequence in the flanking regions [11,48,56-59].
Application of gene editing in selected animal diseases:
Bovine tuberculosis
Using the TALENs technology, the SP110 Nuclear Body Protein (SP110) mouse gene was transferred to the intergenic regions of macrophages, which are the sites of infection expression [47].
The nine exogenous Natural Resistance-Associated Macrophage Protein-1 (NRAMP1) gene's target locations are orthologous and conserved genes lacking linkage disequilibrium. This is because of this NRAMP1 were chosen and inserted using CRISPR/Cas9n into cow foetal fibroblasts that previously derived from naturally resistant intracellular pathogens to M. bovis infection. Cas9 nickase (Cas9n)-mediated single strand breaks were chosen as a superior strategy to prevent issues during NHEJ repair [48]. Transgenic colonies were formed into host NRAMP1 gene because of its well-expressed and M. bovis resistant infection was developed [49,50]. The genes located at the intergenic region between the Actin Beta Gene (ACTB) and the Fascin Actin-Bundling Protein 1 Gene (FSCN1), which affects the length of gene locations on chromosome. The housekeeping genes are consistently located in the FSCN1-ACTB (FA) locus. Exogenous gene silencing by chromatin inactivation was immediately removed [49,60-64].
To prevent the interference relative ratio difference between hspCas9 protein and sgRNA, the SpCas9 (BB)-2A-GFP plasmid was employed for all experiments [48]. The primers intended for each sgRNA cloning. Using Polymerase Chain Reaction (PCR) and Thymine-Adenine (TA) cloning, the target loci from pSpCas9-sgRNA22 and pSpCas9-sgRNA45 transfected in Bovine Fetal Fibroblasts (BFFs) were amplified. Using Sanger sequencing, randomly selected transformed E. coli colonies with indels rates of 21.43% and 41.90%, respectively, were used to demonstrate the existence of Cas9 nuclease-induced indels at the targeted locus. Two nearby BbsI Restriction Enzyme (BbsI) sites were used as target sites for the cloning of multiple sgRNAs. The binding of sgRNA-dCas9 in BFFs, four target sites are present bovine genome with typical cleavage efficiencies and the corresponding sgRNAs were cloned into 3 × FLAG-tagged dCas9 expression vector [65-68]. After the transfection of plasmids into the BFFs until 48 h, the clear reads were aligned with Bos taurus genome sequence using burrows wheeler aligner software [51]. From 200-500 bp strong peaks were intended as target sites of each sgRNA in line with their large numbers of common peaks [47,49].
The on-target and potential off target sites primarily take place in the centres of the binding peaks identified by at 20 bp along with recognition sequences ended with PAM and aligned with sgRNA sequence. The highest density of Cas9 binding was located at the on target site in all four sgRNA groups [52]. The binding of dCas9 to chromatin structure was identified by off-target sites. Large number of common peaks and the Guanine-Cytosine (GG/CC) rich motifs were considered as strong enrichment of 5'-C-Phosphate-G-3' (CpG) islands [52]. The NRAMP1 gene renamed as Solute Carrier Family 11A Member 1 Gene (SLC11A1) is associated with innate resistance to intracellular pathogens [53].
Mastitis
Transgenic cows secreting antimicrobial peptide was proven as mastitis resistance. Somatic cell gene targeting and nuclear transfer jointly enable to produce transgenic animals. ZFN used to induce the exogenous gene of Human Lysozyme (hLYZ) into Β-Casein Gene Locus 2 (CSN2) of Bovine Fetal Fibroblasts (BFF) for integration. The targeted cell clones used as donor cells for Somatic Cell Nuclear Transfer (SCNT) [69,70].
The PCR designed for the targeting vector of β-casein, pCSN2-hLYZ-Neo-GFP was introduced into BFFs together with expression plasmids encoded by ZFNs to create a DSB in intron 2 of CSN2. ZFN cut at unique site at the centre of binding site was converted into a Canonical Type IIP restriction endonucleases (NotI) site to design pTCSN2 vector [71,72]. The exogenous human lysozyme gene and marker genes were injected into the NotI site of pTCSN2 using recombinant DNA techniques. The vector pEGFP-C-hLYZ was designed by injecting human lysozyme gene sequence into multiple cloning site of pEGFP-N1. Matching sequences, β-casein ATG first exon-partial intron 2 and β-casein ATG first exon-partial intron 2-splice acceptor human lysozyme gene sequence were produced by PCR from plasmid pCSN2-hLYZ-Neo-GFP and sub cloned into the vector [73-78].
pEGFP-N1 to construct pEGFP-S-hLYZ
Following transfection procedures into Basic Medical Education Course (BMEC), the transfected cells were all tagged with Enhanced Green Fluorescent Protein (EGFP) fluorescence within 48 hours. The mature pEGFP-C-hLYZ human lysozyme gene now has a kozak site added to it. The EGFP fusion protein in transfected cells was made possible via covert protein secretion [79-82]. Another construct, pEGFP-ShLYZ, was subcloned from the vector pCSN2-hLYZ-Neo-GFP and a CSN2 signal peptide-coding region included as a synthetic intron sequence. Exons of CSN2, translational initiation signal Adenine-Thymine-Guanine (ATG) of β-casein, genomic site cleaved by ZFN, primers used for junction PCR and probe used for southern blotting [83-85]. The predicted size of southern hybridization bands with Restriction Endonuclease Bgl II (BglII) digestion was shown for endogenous and targeted locus of CSN2. The donor plasmid was linked to cleavage location of ZFN pairs and carried 700 bp region of homology to the CSN2 sequence around the cleavage site. ZFNs bind at a specific genomic site leads for the dimerization of FokI nuclease domains [54,86-89].
Porcine Reproductive and Respiratory Syndrome (PRRS)
PRRS virus is infectious viral disease that causes reproductive disorders like premature births, late abortions and dystocia in pigs [55]. Cluster of Differentiation 163 (CD163) was infectious surface receptor sites that allow entry of PRRS virus into porcine alveolar macrophages [90-92]. CD163 is a member of Scavenger Receptor Cysteine-Rich Domain 5 (SRCR5) that expressed at the surface of macrophage which for virus recognition and binding sites. Mutations occurred at this region leads amino acid deletion in 41, 43 and 44 within 5 domains of CD163 [56,57].
In gene edited porcine at exon 7, desirable traits were produced compared with non-edited infected gene piglets. When porcine zygote was edited by CRISPR-Cas9, CD163 and Porcine Aminopeptidase N (pAPN) genes were deleted to generate protein lacking at SRCR5 without any adverse side effects [93,94]. A wild-type control group compared with pigs those take gene editing or CD163 protein lacking pigs not manifested clinical signs like viremia or antibody response that indicating modification made at SRCR5 were resistant against Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) re-infection [1,50]. In the absence of CD163 and pAPN in porcine also were not infected by delta coronavirus [58].
Porcine Epidemic Diarrhoea Virus (PEDV) and Transmissible Gastroenteritis Virus (TGEV)
PEDV is the causative agent of PEDV characterized by infecting all group ages of pigs. Villous enterocytes of small intestine are the predilection site for infection of PEDV. Gene knockout or gene replacement was applied in genetic modification of specific viral receptor gene of pigs that conferred resistance similar to CD163 viral receptor gene for PRRS [59].
Aminopeptidase N (APN), which is a premature stop codon, a vital for PEDV infection, was seen under Knock out (KO) pigs using CRISPR/Cas9 mediated by gene editing and somatic cell nuclear transfer. Gene editing was performed by selecting two candidate gRNAs close to downstream region to the APN start codon. The first and second candidate gRNA was placed in exon 2 and 3 respectively [95-97]. The fetal fibroblasts of pig were transfected with a targeting vector of pX330 containing a hU6-driven gRNA and a humanized Cytosine-Adenine-Guanine (CAG) driven Cas9. Single cell colonies were separately cultured and easily identified by genotyped the positive results [98,99].
The live cloned piglet genotyped was resulted to realize that all of them as biallelic or homozygous KO as free from the frame indels in exon 2 of APN [60]. The null APN pigs were not seen as susceptible for transmissible gastroenteritis virus infection while maintained for PEDV infection. The immunohistochemistry techniques were confirmed the presence of PEDV reactivity and absence of TGEV in APN null pigs. Genome edited pigs was generated lacking APN which confirms, pigs were resistant to TGEV infection. Nonetheless, gene edited animals remained susceptible to PEDV infections [50,59].
The use of gene editing is a modern technology of genetic engineering involving in curing deliberate complicated monogenic diseases and more advantageous by minimizing the expense of medication cost. The presence of gene editing platforms simplifies the understanding of disease pathogenesis for newly emerging and endemic disease in a population. Specific alterations of endogenous base pairs resulted due to homology templates or the integration of engineered donor template providing treatment for complicated forms of disease occurred due to mutations. CRISPR/Cas9 is a recent technique in a simple way of using and cheap cost makes preferred from other techniques. Engineered sgRNA organized from crRNA and a tracrRNA replaces the natural gRNA responsible for directing the Cas9 nuclease of the targeted site to cleave dsDNA in the presence of PAM sequence. The tracrRNA tail, the loop forming scaffold on tracrRNA structure which is useful to enhanced the expression of Cas9 nuclease. CRISPR-Cas9 based zygote edited was lacking CD163 protein did not manifested typical clinical signs like viremia indicated that, modified SRCR5 region were resistant against PRRSV re-infection. Genome edited pigs was generated lacking APN which confirms pigs were resistant to TGEV infection. Nonetheless, gene edited animals for PEDV infections remained susceptible.
CRISPR gene editing was effective in an unexpected some targeted animal disease like the null APN genome edited pigs confirmed as a resistant to TGEV infection but maintained for PEDV infection, therefore to obtain a better result for single genome edited locus simultaneously testing various diseases makes a prolific result.
From starting in earlier gene editing, experimental animals used for preclinical trials causing them by reducing the quality of life, pain, stress and death. But gene edited disease figure as compared to humans very less.
So for, the future monogenetic diseases like protoporphyria, achondroplasia, hypotrichosis and cryptorchidism take into consideration for gene editing.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Menbere E, Tafess K, Ahmed A, Zewude W, Getachew S (2024). Review on the Role of Gene Editing Technology for the Targeted Animal Diseases. J Proteomics Bioinform. 17:669.
Received: 09-Aug-2024, Manuscript No. JPB-24-32816; Editor assigned: 12-Aug-2024, Pre QC No. JPB-24-32816 (PQ); Reviewed: 26-Aug-2024, QC No. JPB-24-32816; Revised: 02-Sep-2024, Manuscript No. JPB-24-32816 (R); Published: 09-Sep-2024 , DOI: 10.35248/0974-276X.24.17.669
Copyright: © 2024 Menbere E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.