Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2024)Volume 12, Issue 10
Background: The treatment of Pulmonary Arterial Hypertension (PAH) and Chronic Thromboembolic Pulmonary Arterial Hypertension (CTEPH) with riociguat is approved and can significantly improve patients' exercise tolerance, hemodynamic parameters, cardiac function, etc. However, for pulmonary arterial hypertension caused by other reasons, the therapeutic effect of riociguat needs further verification. The purpose of this article is to evaluate the therapeutic effect of riociguat on patients with pulmonary arterial hypertension.
Method: A computer was used to conduct literature search on commonly used databases, including EMbase, PubMed, Web of Science, and Cochrane Library, to collect clinical studies related to riociguat and pulmonary arterial hypertension. The search was conducted from database establishment to November 16, 2023 using RevMan 5.4 software for meta-analysis.
Result: Our study included 12 randomized controlled trials. For PAH and CTEPH patients, after receiving riociguat treatment, the 6-Minute Walking Distance (6MWD) was extended by 37.49 meters, with an average reduction of 4.19 mmHg in Pulmonary Arterial Pressure (PAP), 213.53 dynes/second/cm-5 in Pulmonary Vascular Resistance (PVR), 0.75 mmHg in Right Atrial Pressure (RAP), 0.47 l/min/m2 in Cardiac Index (CI), 0.85 l/min in Cardiac Output (CO), 455.31 pg/ml in N-terminal pro-type B Natriuretic Peptide (NT-proBNP), and a reduction in adverse events and clinical deterioration compared to placebo. For other types of Pulmonary Hypertension (PH), including those caused by left heart disease and those caused by lung disease, it has been reported that riociguat extended the 6MWD by 34.9 meters, increased CI by 0.35 l/min, increased CO by 0.67 l/min, and decreased PVR by 41.12 dynes/second/cm-5 compared to placebo. There was no statistically significant difference in other efficacy and safety outcomes among other types of Pulmonary Hypertension (PH).
Conclusion: Our meta-analysis showed that riociguat effectively improved exercise ability and cardiopulmonary hemodynamics in patients with PAH and CTEPH, and was relatively safe and well tolerated for patients with other types of pulmonary hypertension, riociguat treatment may improve their exercise ability, and indicators such as CI, CO, and PVR in cardiopulmonary hemodynamics may also be improved.
Riociguat; Pulmonary arterial hypertension; Chronic thromboembolic pulmonary hypertension; Pulmonary hypertension
PH is a serious pulmonary vascular disease characterized by progressive elevation of PVR and pulmonary arterial pressure, often leading to right heart dysfunction and even death from right heart failure. At present, the latest definition of pulmonary arterial hypertension is the average pulmonary arterial pressure measured by the right heart catheter in a resting state is>20 mmHg, the pulmonary capillary wedge pressure is ≤ 15 mmHg, and the pulmonary vascular resistance is increased by>2 Wood units. The 6th world scientific conference on pulmonary hypertension classified PH into five types based on its different causes, clinical characteristics, and prognosis, including PAH, pulmonary hypertension caused by left heart disease, pulmonary hypertension caused by lung disease and/or hypoxia, pulmonary hypertension caused by chronic thromboembolic pulmonary hypertension and other factors, as well as pulmonary hypertension caused by unclear and/or multifactorial mechanisms [1-7].
In the early stage of pulmonary hypertension, there are no specific clinical manifestations, and the vast majority of patients have significantly delayed treatment times. At least 1/5 of patients take more than 2 years from the onset of symptoms to diagnosis. Some patients with pulmonary hypertension may only present with underlying disease-related symptoms in the early stages, and right heart failure symptoms may occur when pulmonary artery pressure significantly increases.
The most common symptom of pulmonary hypertension is post activity shortness of breath, while other symptoms include fatigue, dizziness, chest pain, chest tightness, palpitations, blackness, syncope, etc.
At present, the treatment of pulmonary hypertension is based on supportive therapy and a diversified and precise combination therapy of multiple targeted drugs. In addition to supportive treatment measures such as oral anticoagulants, oxygen therapy, diuresis, digoxin, and iron supplementation, patients should also undergo general treatment measures such as contraception, rehabilitation and exercise training, elective surgery selection, infection prevention, psychological support, and avoiding highaltitude or low oxygen environments. If necessary, surgical treatment can also be performed.
PH targeted drugs include endothelin receptor antagonists, type 5 phosphodiesterase inhibitors, prostacyclin drugs, guanylate cyclase agonists, etc. Riociguat is a new type of soluble guanylate cyclase agonist that can independently or synergistically increase plasma cyclic Guanosine Monophosphate (cGMP) levels. Riociguat is currently the only targeted drug with dual indications for PAH and CTEPH, and its therapeutic effect on other types of pulmonary arterial hypertension is not yet clear. The main purpose of this article is to evaluate and study the therapeutic effect of riociguat on patients with pulmonary arterial hypertension [8].
Selection criteria
Eligibility criteria: (1) Randomized Clinical Trials (RCTs); (2) The study population comprised patients with PH, no matter what type of PH and (3) Intervention treatment included riociguat versus placebo.
Exclusion criteria: (1) Duplicated data; (2) Sub-study of the RCT; (3) Ongoing trials and (4) Studies were excluded if the research had been conducted on infants.
Search strategy
Using computers to conduct literature searches on commonly used databases, including EMbase, PubMed, Web of Science, Cochrane library, to collect research related to riociguat (BAY 63- 2521), placebo, PAH, CTEPH, and PH. The search was conducted from the start of database construction until November 16, 2023. The retrieval method of this article is a combination of free words and topic words.
Study selection and data collection
Two researchers independently screened and extracted relevant information from the literature included in this article, and then cross checked each other.
If there is any disagreement in the opinion of that literature, we consulted a third party for assistance in judgment. Some literature lacks relevant outcome indicator data, and we tried to contact the author as much as possible to supplement it..
The main extracted data include: General information of the included studies, number of cases of study subjects, 6MWD, PVR, PAP, Pulmonary Capillary Wedge Pressure (PCWP), NT-proBNP, Mean Arterial Pressure (MAP), RAP, CO, mean Pulmonary Artery Pressure (mPAP), CI, Systemic Vascular Resistance (SVR), Mixed Venous oxygen Saturation (SvO2), Diagnostic Pressure Gradient (DPG), scores on the EuroQol group 5-Dimension self-report questionnaire (EQ-5D score), scores on the Living with Pulmonary Hypertension (LPH) score questionnaire (an adaptation of the minnesota living with heart failure questionnaire) and adverse reactions, etc. As to missing or unclear information, we tried to contact original trial researchers by phone or email.
Assessment of risk of bias
For each study included in this article, two authors independently evaluated using the criteria outlined in the Cochrane intervention system review manual. Bias assessment includes random sequence generation, allocation concealment, participant and personnel blinding, incomplete outcome data, outcome evaluation blinding, selective outcome reporting, and other biases. The risk of bias is divided into low risk, high risk, and unclear risk. All differentials were resolved by consensus [9].
Statistical analysis
Perform statistical analysis using RevMan 5.4 software; for binary variables, the Odds Ratio (OR) and 95% CI are used to represent the size of the effect; continuous variables are represented using Mean Difference (MD) or Standardized Mean Difference (SMD) and a 95% confidence interval.
Heterogeneity I2 statistics included in the study were evaluated, and I2 values greater than 50% were considered to have heterogeneity; I2 value<50% is analyzed using a fixed effects model, I2 value>50% is analyzed using a random effects model, sensitivity analysis was conducted by identifying each included study individually, and performing a summary analysis on the remaining studies to assess where any single included study had an exceptional impact on the results of the entire meta-analysis.
Study outcomes
Indicators for evaluating efficacy include: (1) Changes in 6MWD indicates the change in walking distance within 6 minutes from baseline to the end of the stud; (2) Cardiopulmonary hemodynamic parameters: PVR, PAP, MAP, RAP, CO, PCWP, mPAP, CI, SVR, DPG, SvO2, and NT-proBNP and (3) Survey questionnaire-LPH score (range from 0 to 105, with higher scores indicating worse quality of life) and EQ-5D score (range from –0.6 to 1.0, with higher scores indicating a better quality of life).
Indicators for evaluating security include: (1) Advanced events included but were not limited to headache, dysopia, peripheral edema, nausea, dizziness, diarrhoea and syncope; (2) Clinical worsening clinical worrying covered hospitalization due to PH, initiation of new treatment for PH, increase in 6MWD due to PH, persistent worrying of World Health Organization (WHO) functional status due to PH, and death [10,11] (Table 1).
| Trial | Etiology | Functional class | Riociguat dose (mg, tid) | Follow up time (weeks) | Riociguat 2.5mg | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbers (n) | Age (years) | Female(n) | BMI (kg/m2) | 6-MWD (m) | Numbers (n) | Age (years) | F | BMI (kg/m2) | 6-MWD (m) | |||||
| Ghofrani et al. [12] | PAH | II/III | 2.5 | 12 | 254 | 51±17 | 203 | 26±5 | 361±68 | 126 | 51±17 | 98 | 26±6 | 368±75 |
| Rosenkranz et al. [13] | PAH-CHD | II/III | 1.5-2.5 | 12 | 15 | 35±14 | 13 | 21±2 | 369±17 | 12 | 40±16 | 10 | 24±3 | 360±59 |
| Galie et al. [14] | PAH | II/III | 2.5 | 12 | 123 | 48±17 | 94 | 25±5 | 370±66 | 66 | 48±18 | 52 | 26±6 | 360±80 |
| Galie et al. [14] | PAH | II/III | 2.5 | 12 | 131 | 54±15 | 109 | 27±6 | 353±69 | 60 | 53±15 | 46 | 27±6 | 376±68 |
| Ghofrani et al. [12] | CTEPH | II/III | 0.5-2.5 | 16 | 173 | 59±14 | 118 | 27±6 | 342±82 | 88 | 59±13 | 54 | 28±5 | 356±75 |
| Kim et al. [15] | CTEPH | II/III | 0.5-2.5 | 16 | 121 | 59±14 | 86 | 27±5 | 335±83 | 68 | 60±12 | 45 | 28±5 | 351±75 |
| Kim et al. [15] | CTEPH | II/III | 0.5-2.5 | 16 | 52 | 60±14 | 32 | 28±7 | 360±78 | 20 | 57±15 | 9 | 28±6 | 374±72 |
| Bonderman et al. [16] | PH-LHD | II/III | 2 | 6 h-24 h | 10 | 72.8 (59.0–83.0) | 5 | 29.3 (23.5-33.4) | NR | 11 | 30.2 (21.8-36.0) | 6 | 55 | NRS |
| Bonderman et al. [17] | PH-LHD | II/III | 2 | 16 | 67 | 59.3 (26.0–76.0) | 12 | 28.9±4.9 | 380.9±126.1 | 69 | 58.9 (25.0-79.0) | 8 | 28.7±5.8 | 382.1±123.8 |
| Nathan et al. [18] | LD-PH | II/III/IV | 0.5-2.5 | 26 | 73 | 68±8 | 50 | 30±5 | 307±80 | 74 | 69±8 | 45 | 28±6 | 324±66 |
| Dachs et al. [19] | PH-HFpEF | II/III | 0.5-1.5 | 26 | 58 | 70.6±8.0 | 46 | 32.1±6.4 | 308.83±109.41 | 56 | 72.1±8.5 | 37 | 30.3±6.4 | 342.57±116.14 |
| Baughman et al. [20] | sarcoidosis and SAPH | NR | 0.5-2.5 | 48 | 8 | 52±7.0 | 6 | NR | 271±95.8 | 8 | 64±6.3 | 8 | NR | 332±66.7 |
| Ghofrani et al. [21] | PAH | II/III | 2.5 | 12 | 42 | 55±15 | 36 | NR | 348±80 | 14 | 53±18 | 12 | NR | 363±70 |
| Ghofrani et al. [21] | PAH | II/III/IV | 2.5 | 12 | 71 | 55±15 | 60 | NR | 360±62 | 40 | 53±15 | 30 | NR | 385±61 |
| Wang et al. [22] | PAH | I/II/III | 2.5 | 12 | 43 | 38±13 | 34 | NR | 382±51 | 24 | 41±16 | 18 | NR | 387±50 |
| Wang et al. [22 ] | CTEPH | I/II/III | 2.5 | 16 | 21 | 47±10 | 14 | NR | 375±57 | 11 | 52±13 | 8 | NR | 364±85 |
Note: BMI: Body Mass Index; PAH-CHD: Pulmonary Arterial Hypertension-Congenital Heart Disease; LD-PH: Left heart Disease-Pulmonary Hypertension; NR: Not Reported; SAPH: Sarcoidosis Associated Pulmonary Hypertension; PAH: Pulmonary Arterial Hypertension; CTEPH: Chronic Thromboembolic Pulmonary Hypertension.
Table 1: Characteristics of studies included in the meta-analysis.
Each database was strictly searched according to the retrieval strategy, and a total of 5099 relevant literature were obtained. After step-by-step screening, a total of 12 clinical studies were included in this study and main characteristics of the included studies are described in Table 1. Ghofrani et al., and Kim et al., analyzed the same CHEST-1 data. Kim et al., divided the experimental group of patients into two groups: Inoperable CTEPH and persistent/ recurrent PH following PEA. Ghofrani et al., analyzed both primary and secondary end points and in hemodal variables, while Kim et al., analyzed both [12,14,15]. Both studies were included as the group analyzed the hemodynamic parameters for two groups of patients; the data analyzed by Ghofrani et al., and Galie et al., were both from the patent study. Galie et al., divided the experimental group (2.5 mg) of patients into treatment naïve and pre created overall groups, with the main outcome measure being the hemodynamic parameters data; Ghofrani et al., mainly reported the primary and secondary end points and in hemodynamic variables indicators of the 2.5 mg group and placebo group [12,14,21]. Therefore, the two articles complement each other in terms of outcome indicators, and both articles are included in this study and each study targets a specific population of patients, so they are all included in the study. The process and results of literature screening are shown in Figure 1. Among the included patients, they were randomly divided into a treatment group and a control group. All trials were followed up for more than 12 weeks, with a follow-up time of 48 weeks by Baughman et al. [12-23] (Figure 1).
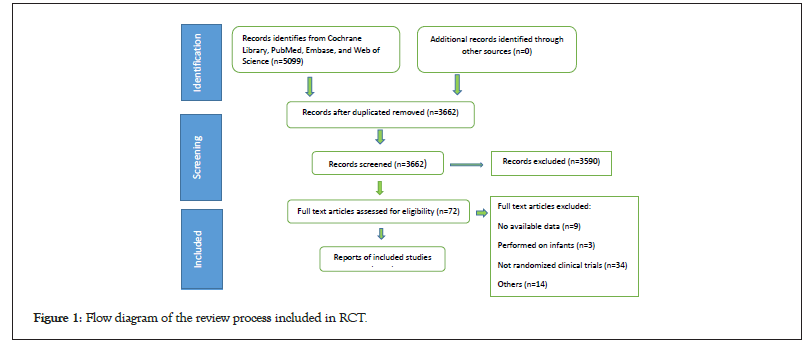
Figure 1: Flow diagram of the review process included in RCT.
Results of bias risk evaluation for the enrolled RCTs are shown in Figure 2. Green represents low risk, red represents high risk, and yellow represents unclear risk and can be seen that there was no obvious bias in this study. All trials had a withdrawal of<10% at the time of publication of the outcomes of interest in our analysis (Figure 2).
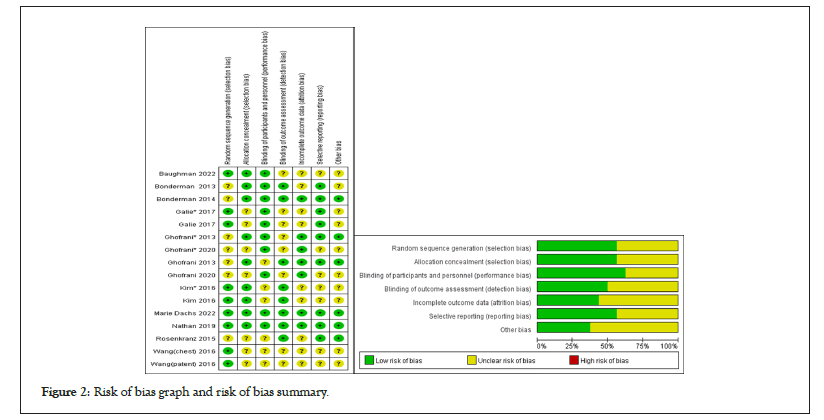
Figure 2: Risk of bias graph and risk of bias summary.
Meta-analysis
• The 6MWD change was included in a total of 9 clinical studies, including 5 studies on PAH and CTEPH, and 4 studies on other types of pulmonary hypertension. Subgroup analysis was conducted based on different classifications of pulmonary hypertension, and the results showed that patients in the PAH and CTEPH groups had an increase of 37.49 meters in 6MWD (p<0.00001), while those in the other types had an increase of 34.9 meters (p=0.05). At a 95% CI , the three studies of Bonderman et al., Nathan et al. and Dachs et al., showed no statistically significant difference between the two groups, with only the Baughman et al., study showing riociguat treatment can increase the exercise endurance of patients with other types of pulmonary hypertension [16,18- 20] (Figure 3).
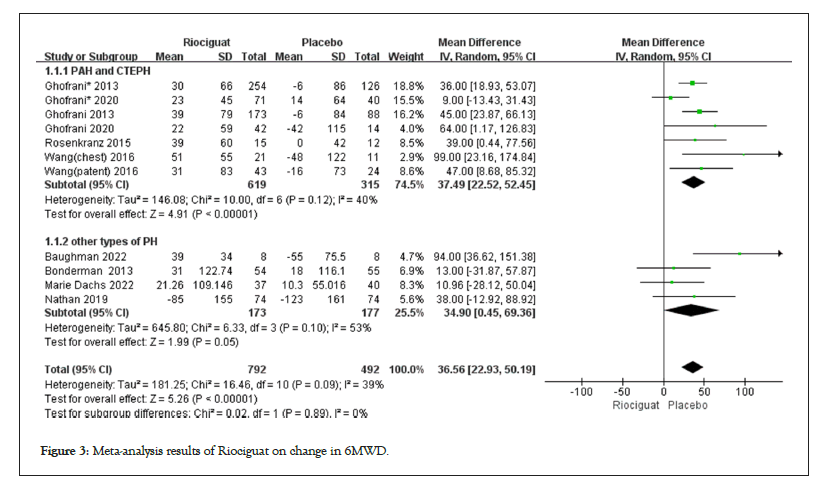
Figure 3: Meta-analysis results of Riociguat on change in 6MWD.
• Cardiopulmonary hemodynamic parameters includes 1. The treatment with riociguat showed statistical significance compared to the control group in terms of CI, CO, and PVR. In PAH and CTEPH, riociguat treatment increased CI by 0.47 L/min/m2, CO by 0.85 l/min, and PVR by -213.53 dyn/s/cm5; in patients with other types of pulmonary hypertension, the CI increased by 0.35 l/min/m2, the CO increased by 0.67 l/min, and the PVR decreased by -41.12 dyn/s/cm5 (Figures 4-6); 2. The indicators of MAP, mPAP, NT-proBNP, RAP, SVR, DPG, and PAP, as well as the comparison between riociguat treatment and placebo, have statistical significance in PAH and CTEPH patients, but there is no statistical significance in the comparison between the two groups in other types of pulmonary hypertension patients; 3. The SvO2 results showed (3.71; 2.78, 4.65; p for effect<0.00001; I2=48%; p for heterogeneity=0.05); no subgroup analysis was conducted (Table 2); 4. There was no statistically significant difference between the two groups in terms of PCWP in patients with PAH, CTEPH, and other types of pulmonary hypertension (Table 2 and Figures 4-6).
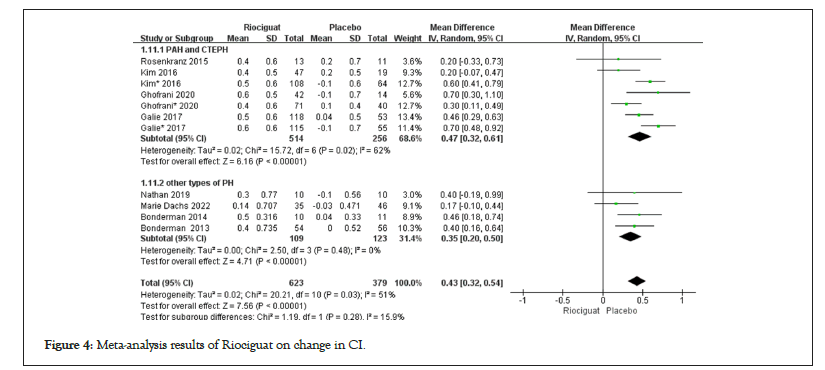
Figure 4: Meta-analysis results of Riociguat on change in CI.
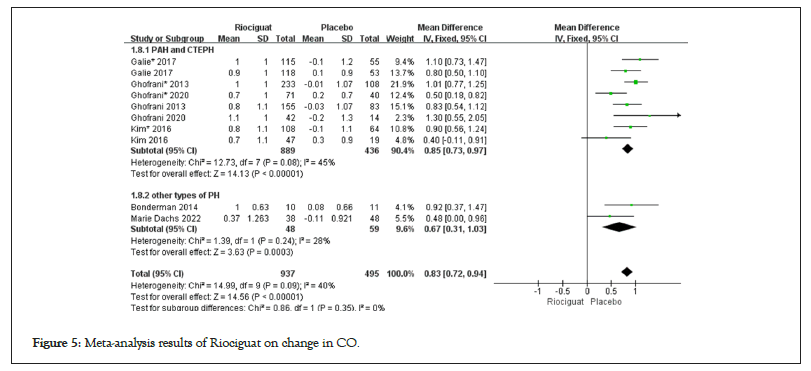
Figure 5: Meta-analysis results of Riociguat on change in CO.
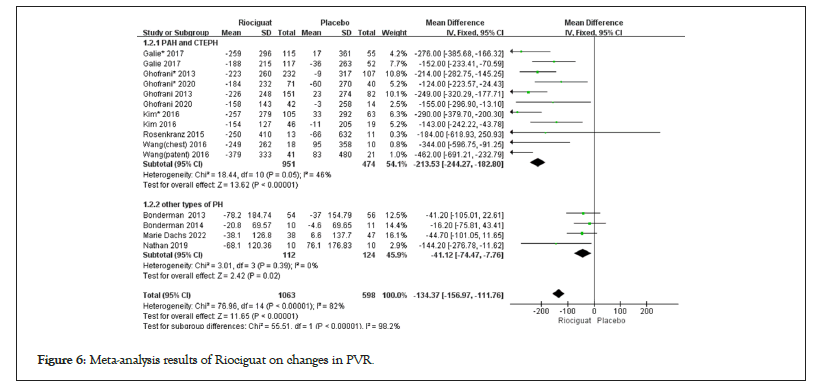
Figure 6: Meta-analysis results of Riociguat on changes in PVR.
• The EQ-5D score (0.1; 0.05,0.16; p for effect=0.0002; I2=22%; p for heterogeneity=0.27) showed that riociguat treatment improved the scores of PAH and CTEPH patients, while other types of pulmonary hypertension did not show significant improvement in EQ-5D score (p=0.54). The LPH score results showed (-6.73; -9.49, -3.97; p for effect<0.00001; I2=0%; p for heterogeneity=0.7) that the riociguat treatment group had statistical significance compared to the placebo group; the included studies did not analyze patients with other types of pulmonary hypertension (Table 2).
| Outcome indicators | Effect mode | PAH and CTEPH | Other types of PH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies included | Heterogeneity test results | Meta-analysis results | Number of studies included | Heterogeneity test results | Meta-analysis results | ||||||
| p-value | I2 value (%) | RR (95% CI) | p value | p value | I2 value (%) | RR (95% CI) | p-value | ||||
| MAP | Fixed | 6 | 0.74 | 0 | -7.83 (-9.2, -6.46) | <0.00001 | 2 | 0.57 | 0 | -1.84 (-4.36, 0.68) | 0.15 |
| mPAP | Fixed | 4 | 0.44 | 0 | -3.7(-4.77, -2.63) | <0.00001 | 3 | 0.26 | 25 | -0.94 (-3.51, 1.63) | 0.47 |
| NT-proBNP | Fixed | 4 | 0.16 | 40 | -455.31 (-638.25, -272.37) | <0.00001 | 2 | 0.58 | 0 | -16.6(-79.31, 46.12) | 0.6 |
| RAP | Fixed | 6 | 0.7 | 0 | -0.75 (-1.3, -0.21) | 0.007 | 3 | 0.76 | 0 | 0.11(-1.25, 1.47) | 0.87 |
| SVR | Random | 4 | 0.01 | 64 | -345.96 (474.4, -217.52) | <0.00001 | 2 | 0.14 | 55 | -147.41(-342.72, 47.90) | 0.14 |
| DPG | Random | 1 | 0.22 | 32 | -3.97 (-6.55,-1.38) | 0.003 | 1 | - | - | -0.7(-3.5, 2.1) | 0.62 |
| PAP | Random | 1 | 0.36 | 0 | -4.19 (-5.59, -2.8) | <0.00001 | 1 | - | - | 0.77 (-1.6,3.14) | 0.53 |
| SvO2 | Fixed | 6 | 0.05 | 48 | 3.71 (2.78, 4.65) | <0.00001 | 0 | - | - | - | - |
| PCWP | Fixed | 3 | 0.75 | 0 | 0.54 (-0.07, 1.15) | 0.08 | 2 | 0.5 | 0 | -0.58 (-2.77,1.61) | 0.6 |
| EQ-5D score | Random | 4 | 0.27 | 22 | 0.1 (0.05, 0.16) | 0.0002 | 2 | 0.15 | 51 | 1.67 (-3.73,7.07) | 0.54 |
| LPH score | Fixed | 4 | 0.7 | 0 | -6.73(-9.49, -3.97) | <0.00001 | 0 | - | - | - | |
Table 2: Summary of studies analyzing the effects of Riociguat in PAH and CTEPH patients.
Safety and adverse reactions
The results of advanced events and riociguat treatment in PAH and CTEPH patients showed no increase in advanced events compared to the control group (1.46; 1.04,2.06; p for effect=0.03; I2=0%; p for heterogeneity=0.49); In other types of pulmonary hypertension patients, the results showed (1.44; 0.73,2.85; p for effect=0.30; I2=10%; p for heterogeneity=0.34), and there was no statistically significant difference between the two groups. Clinical worthing all events (0.25; 0.11; 0.54; p for effect=0.0004; I2=0%; p for heterogeneity=0.94) (Figures 7 and 8).
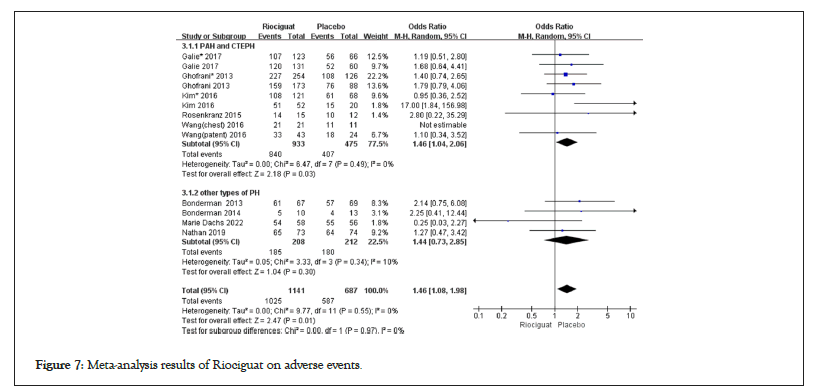
Figure 7: Meta-analysis results of Riociguat on adverse events.
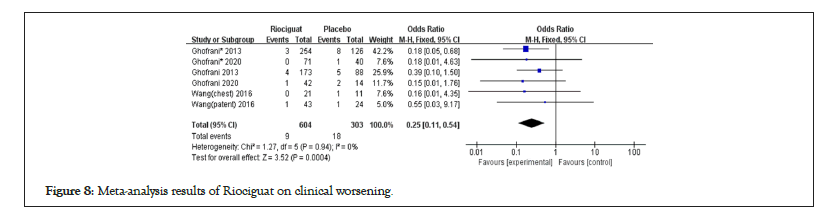
Figure 8: Meta-analysis results of Riociguat on clinical worsening.
In this meta-analysis enrolled in 12 randomized trials, main findings could be summarized as follows: Riociguat effectively improved the exercise ability and cardiopulmonary hemodynamics of patients with PAH and CTEPH, and was relatively safe and well tolerated; for patients with other types of pulmonary hypertension, riociguat treatment may improve their exercise ability, and indicators such as CI, CO, and PVR in cardiopulmonary hemodynamics may also be improved.
The 6MWD test can be used to evaluate patients' exercise tolerance, medical intervention effectiveness, and disease prognosis. This experiment is very simple and practical, with high safety and convenience. This meta-analysis analyzed 9 studies on the 6MWD, with 5 studies on patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. The results of riociguat treatment showed a significant improvement in patient exercise endurance, with a 37.49 meter increase in 6MWD compared to baseline. Among them, 4 studies focused on pulmonary hypertension related to left heart, sarcoidosis, and connective tissue disease. Bonderman et al., and Dachs et al., showed left heart related pulmonary hypertension, and Nathan et al., showed idiopathic interstitial pneumonia related pulmonary hypertension. There was no statistically significant difference in the results of the 6MWD test between the two groups in these three studies Baughman et al., [17-20]. The results of sarcoidosis and Sarcoidosis Associated Pulmonary Hypertension (SAPH) (excluding patients with significant emphysema in this study) showed that treatment with riociguat can increase walking distance by six minutes. Riociguat is effective in treating patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension, and it is believed to be related to the similarity in hemodynamics and pathophysiology between these two types of patients. The vascular remodeling of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension mainly occurs in the middle and small arteries of the lungs, while the pulmonary vascular remodeling of Pulmonary Hypertension due to Left Heart Diseases (PH-LHD) mainly occurs in the pulmonary veins [24-26].
Cardiopulmonary hemodynamic parameters
CI, CO, and PVR have statistical significance in the comparison between the two groups and subgroups; CO is a comprehensive indicator reflecting the function of the heart pump, which has a significant decrease in low output heart failure; If the heart index is higher than normal, it indicates a high tissue metabolic rate, fast blood flow, and a decrease in heart index, which may lead to severe heart failure; PVR mainly refers to the obstruction of blood flow by the pulmonary blood vessels, mainly due to the decrease in elasticity of the blood vessels that remain in the pulmonary blood vessels. The above indicators can all be used as indicators to evaluate right heart function. Riociguat is a soluble Guanylate Cyclase (sGC) agonist, which is an important signaling enzyme that can be activated by NO to catalyze the synthesis of cGMP, known as the classic NO sGC cGMP signaling pathway. NO−sGC−cGMP signaling can be dysregulated in several ways in cardiovascular, cardiopulmonary and cardiorenal diseases including PAH and other forms of PH. Patients with pulmonary arterial hypertension have insufficient synthesis of NO, and although NO donor drugs are effective, their half-lives are short. Riociguat is a soluble guanylate cyclase agonist with a dual mechanism of action. On the one hand, it can directly bind to the form of sGC containing hemoglobin, stabilizing the enzyme in its active structural conformation, independent of NO binding and can directly activate sGC without relying on NO; on the other hand, they make sGC more sensitive to endogenous NO by stabilizing the NO heme complex thereby achieving anti fibrotic, vasodilatory, anti-proliferative, and anti-inflammatory effects [27- 32].
Our meta-analysis showed that riociguat effectively improved the exercise ability and cardiopulmonary hemodynamics of PAH and CTEPH patients, and was relatively safe and well tolerated; for patients with other types of pulmonary hypertension, riociguat treatment may improve their exercise ability, and indicators such as CI, CO, and PVR in cardiopulmonary hemodynamics have been improved.
• The sample size of the study is small, especially for patients with other types of pulmonary hypertension.
• As this meta-analysis is not based on patient-level data, it shares the possible shortcomings of the original articles.
• We did not conduct subgroup analysis on different classifications of pulmonary arterial hypertension; in the fourth study, the dosage and observation/follow-up time of patients taking riociguat were inconsistent.
• There was inconsistency in the dosage of Riociguat and follow-up times among the included studies.
• The high level of heterogeneity among the included studies, particularly regarding patient characteristics and treatment regimens, may limit the generalizability of the findings and complicates the interpretation of the overall treatment effect.
• Finally, while the review highlights short-term outcomes, the long-term safety and efficacy of Riociguat remain inadequately explored, as many studies had relatively short follow-up periods, emphasizing the need for more comprehensive longterm data, especially concerning adverse events such as liver toxicity and drug interactions.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Dang L, Zhang Y, Mou X, Shu T, Cao M, Chu Ai-Ai (2024). Riociguat for the Treatment of Pulmonary Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Angiol Open Access. 12:516.
Received: 30-Sep-2024, Manuscript No. AOA-24-34883; Editor assigned: 03-Oct-2024, Pre QC No. AOA-24-34883 (PQ); Reviewed: 17-Oct-2024, QC No. AOA-24-34883; Revised: 24-Oct-2024, Manuscript No. AOA-24-34883 (R); Published: 31-Oct-2024 , DOI: 10.35841/2329-9495.24.12.516
Copyright: © 2024 Dang L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.