Journal of Hepatology and Gastrointestinal disorders
Open Access
ISSN: 2475-3181
ISSN: 2475-3181
Research Article - (2021)Volume 7, Issue 3
Background: Portal vein thrombosis (PVT) is a common complication of liver cirrhosis. PVT impact on disease progression is not clarified as yet. Anticoagulation therapy is considered effective in this setting, but is associated with potentially bleeding episodes.
Aim: to assess the risk factors and clinical impact of non-neoplastic PVT complicating cirrhosis, as well as the treatment profile and its efficacy in clinical practice.
Methods: A retrospective monocentric study over a period of 19 years including all patients diagnosed with cirrhosis and non-neoplastic PVT was conducted.
Results: A total of 49 patients were enrolled in the present study. The mean age was 60.86±11.61 years old. Chronic viral hepatitis was the most frequent cause of cirrhosis (63.2%). Most of our cases had advanced liver disease (89.9% Child class B/C) with a mean MELD score of 19.27. The risk factors for thrombophilia, inherited or acquired, were: a deficiency in coagulation inhibitors either isolated or combined (protein S, protein C and anti-thrombin III) in 19 patients, a heterozygous Factor V Leiden mutation in 2 patients, a heterozygous MTHFR mutation in one patient, an antiphospholipid antibodies syndrome in 2 patients, an essential thrombocythemia in one patient. Anticoagulant therapy was indicated in half of the cases. Multivariate analyses demonstrated that thrombus extension was the only independent predictive factor of portal vein recanalization (p=0.009). During follow-up, progression was observed in 8% of treated patients with anticoagulants versus 12.5% of untreated patients (p=0.12). Our study has shown that anticoagulant treatment is not associated with elevated risk of bleeding or developing other complications. The mean survival was higher in patients treated successfully (38.31 months vs. 23.41 months, p=0.204).
Conclusion: Our outcomes confirm that anticoagulation therapy in cirrhotic patients with non-neoplastic PVT is not associated with increased risk of liver disease decompensation, including bleeding.
Cirrhosis; Portal vein thrombosis; Anticoagulation therapy
Portal vein thrombosis (PVT) is defined by the formation of a thrombus within the portal vein trunk and intrahepatic portal branches [1]. The pathophysiological mechanism of non-tumor PVT in cirrhotic patients is not clearly defined [2]. The presence of a prothrombotic abnormalities could be counterbalanced by a chronic hypo coagulant state linked to the alteration of the synthesis of procoagulant factors, linked to hepatic insufficiency [3,4]. Cruoric thrombosis in cirrhosis is a relatively common complication, with a prevalence reported at 11.2% by Amitrano et al. [5] and variant between 10 and 15% in the literature [6,7].
The risk factors for the constitution of PVT are difficult to specify. Indeed, in cirrhotic patients, local factors (decrease in portal flow, architectural changes in the liver due to endothelial damage) seem to play an important role. Nevertheless, some authors have demonstrated in these patients a higher frequency of genetic mutations such as mutations in prothrombin and MTHFR (Methylene Tetrahydrofolate Reductase) [8]. Other coagulation disorders, either inherited or acquired, could also be additional cofactors, logically with the multifactorial nature of thrombosis [9]. PVT is considered an event of poor prognosis. However, the aggravating role of PVT in the progression of cirrhosis remains uncertain [10]. At the same time, the optimal management of PVT in the context of cirrhosis is not clearly defined in the literature, and anticoagulant treatment should always be considered with caution in patients at risk of bleeding especially from gastrointestinal tract caused by portal hypertension [5,6].
The aim of our study was to determine the risk factors for the onset of PVT in cirrhotic patients, to study its impact on the course of cirrhosis and to evaluate the effect of anticoagulant therapy on portal recanalization, the occurrence of complications and survival.
We conducted a retrospective monocentric study that included all patients diagnosed with cirrhosis and non-neoplastic PVT in the gastroenterology department of Military Hospital of Instruction of Tunis over a period of 19 years from January 2000 to December 2018.
Exclusion criteria
We excluded patients with a history of progressive neoplastic pathology or in remission at the time of diagnosis of PVT. Hepatocellular carcinoma either at the time or before the diagnosis of PVT.
Patients under anticoagulants for an etiology other than PVT before their inclusion, with a follow-up of less than six months and cirrhotic patients who developed hepatocellular carcinoma during the follow-up period within 6 months of PVT were excluded from the study.
For each patient, epidemiological data, risk factors, clinical, biological, imaging, endoscopy, therapeutic and evolutionary data were collected.
The therapeutic data concerned the indications for anticoagulant treatment and the therapeutic modalities: Type of treatment; Low Molecular Weight Heparin (LMWH) or Anti-Vitamin K (AVK) and duration of treatment.
Statistical analysis
Descriptive analytic analysis was performed. In order to assess the effect of anticoagulant therapy on portal recanalization, the course of cirrhosis and survival, a comparative study was conducted between the treated and untreated group of patients.
• Group 1: Patients treated.
• Group 2: Untreated patients.
Forty-nine cases of non-tumor PVT occurring in cirrhotic patients at an advanced stage of the disease (Child B or C in 89.9% of cases) were included. The mean age at diagnosis was 60.86±11.61 years, predominantly male (sex ratio M/W: 1.13).
The diagnosis of PTV was made in 34.7% of cases during the first two to three years following the diagnosis of cirrhosis. PTV was classified as acute in 53.1% of cases. It was partial in 38.8% of patients. Signs of endoscopic portal hypertension were present in 91.8% of patients. The patient’s characteristics and laboratory findings at the time of diagnosis are summarized in Tables 1 and 2.
| Parameters | Patients |
|---|---|
| Age (years) | 60,86 |
| Gender: Man/Woman | 1.13 |
| Diabetes | 21 |
| Hypertension | 13 |
| Dyslipidemia | 8 |
| Ischemic heart disease | 4 |
| Hepatitis B cirrhosis | 11 |
| Hepatitis C cirrhosis | 20 |
| Non alcoholic steatohepatitis | 7 |
| Auto-immune hepatitis | 3 |
| Cryptogenic cirrhosis | 8 |
| Child Pugh A | 5 |
| Child Pugh B | 37 |
| Child Pugh C | 7 |
| Esophageal varices | 45 |
| Gastric varices | 24 |
| Hypertensive gastropathy | 22 |
| History of endoscopic ligation of esophageal varices | 9 |
| Refractory ascites | 7 |
| History of digestive bleeding | 7 |
| Spontaneous Bacterial Peritonitis | 3 |
| Hepatic encephalopathy | 3 |
Table 1: Patient characteristics.
| Parameters | Mean ± standard deviation | Extreme |
|---|---|---|
| Total bilirubin (µmol/l) | 43.1 ± 33.2 | 12-217 |
| Creatinine (µmol/l) | 81.91 ± 26.31 | 43-203 |
| Albumine (g/l) | 31.08 ± 3.25 | 22-36 |
| Prothrombin (%) | 59.8 ± 7.199 | 39-73 |
| International normalized ratio (INR) | 1.35 ± 0.17 | 1-1,75 |
| Factor V | 41.27 ± 10.715 | 32-65 |
| Platelets | 98485 ± 87079 | 25000-598000 |
Table 2: Laboratory findings at the time of diagnosis.
The risk factors for PTV found in our study were a deficiency of coagulation inhibitors, either isolated or associated (proteins C, S and Antithrombin III) in 19 patients, a heterozygous MTHFR mutation in one patient, a heterozygous mutation of factor V in two patients, Anti-Phospholipid Syndrome (APS) in two patients, Essential Thrombocythemia (ET) in one patient and Multiple Myeloma (MM) in one case (Tables 3 and 4).
| Constitutional thrombophilia | n | % |
|---|---|---|
| Protein S deficiency | 6 | 12.2 |
| Protein C deficiency | 3 | 6.1 |
| Protein S and C deficiency | 6 | 12.2 |
| Antithrombin and Protein C deficiency | 2 | 4.1 |
| Antithrombin and Protein S deficiency | 2 | 4.1 |
| Factor V Leiden heterozyous mutation | 2 | 4.1 |
| Hyperhomocysteinemia | 1 | 2.04 |
| Heterozyous mutation MTHFR | 1 | 2.04 |
Table 3: Results of the constitutional thrombophilia workup.
| Acquired thrombophilia | Bilan made | % | Results | % |
|---|---|---|---|---|
| Antiphospholipid syndrome | 34 | 69.4 | 2 | 4.1 |
| Myeloproliferative disease | ||||
| JAK2 | 9 | 18.4 | 1 (Essential thrombocythaemia) | 2 |
| Bone marrow biopsy | 19 | 38.8 | 1 (Multiple myloma) | 2 |
| Myelogram | 1 | 2 | 0 | 0 |
| Paroxysmal nocturnal haemoglobinuria | ||||
| Ham test | 3 | 6.1 | 0 | 0 |
| Flow cytometry | 12 | 24.5 | 0 | 0 |
Table 4: Results of the acquired thrombophilia assessment.
Anticoagulant therapy was indicated in 51% of cases. The mean duration of treatment was 16.14 ± 11.48 months. The patients were treated with AVK in 20 cases and with LMWH in five cases (Table 5).
| Parameters | G1 (n=25) | G2 (n=24) | p |
|---|---|---|---|
| Age (years) | 58,72 | 63,08 | 0.207 |
| Sex-ratio | 14-11 | 12-12 | 0.674 |
| Smoking | 8 | 8 | 0.921 |
| Ethylism | 5 | 4 | 1 |
| Pathological history | |||
| Diabetes | 13 | 8 | 0.187 |
| Hypertension | 8 | 5 | 0.376 |
| Dyslipidemia | 4 | 4 | 1 |
| Cardiopathies | 3 | 1 | 0.609 |
| History of deep vein thrombosis | 4 | 0 | 0.11 |
| Hepatitis B cirrhosis | 5 | 6 | 0.675 |
| Hepatitis C cirrhosis | 8 | 12 | 0.2 |
| Auto-immune hepatitis | 2 | 1 | 1 |
| Non alcoholic steatohepatitis | 4 | 3 | 1 |
| Cryptogenic cirrhosis | 6 | 2 | 0.702 |
| History of digestive bleeding | 5 | 2 | 0.417 |
| Refractory ascites | 4 | 3 | 1 |
| Hepatic encephalopathy | 3 | 0 | 0.235 |
| Spontaneous Bacterial Peritonitis | 0 | 3 | 0.11 |
| Average CHILD score | 8,24 | 7,66 | 0.095 |
| Average MELD score | 20,36 | 18,13 | 0.225 |
| MELD score >15 | 15 | 11 | 0.321 |
| PTV | |||
| Acute | 16 | 10 | 0.117 |
| chronic | 9 | 14 | |
| PTV | |||
| partial | 13 | 6 | 0.052 |
| complete | 12 | 18 | |
| Extension of thrombosis | 18 | 7 | 0.003 |
| Esophageal varices | 22 | 23 | 0.609 |
| Gastric varices | 8 | 7 | 0.83 |
| Hypertensive gastropathy | 10 | 12 | 0.482 |
| History of endoscopic ligation of esophageal varices | 8 | 1 | 0.023 |
Note: Group 1: Patients treated; Group 2: Untreated patients; PTV: Portal vein thrombosis.
Table 5: Comparative study of the characteristics of treated and untreated patients.
Under treatment, vascular permeabilization was successful in 13 patients (52%) with an average time to recanalization (complete or partial) of 9.66±10.16 months. Despite the absence of anticoagulant treatment, spontaneous recanalization was noted in 5 patients (20.83%). The number of repermeabilized thromboses was significantly higher in univariate analysis when anticoagulant treatment was indicated (p=0.024) (Table 6). In multivariate analysis, only the extent of PTV was independent predictors of portal vein repermeabilization (Table 7). Progression was observed in 2 treated patients (8%) versus 3 untreated patients (12.5%) without this difference being statically significant (p=0.12).
| Parameters | G1 (n=25) | G2 (n=24) | p |
|---|---|---|---|
| The initiation of anticoagulant therapy | 13 | 5 | 0.024 |
| Acute PTV | 9 | 5 | 0.008 |
| Partial PTV | 9 | 4 | <0.001 |
| Extensive PTV | 13 | 3 | <0.001 |
Note: Group 1: Patients treated; Group 2: Untreated patients; PTV: Portal vein thrombosis.
Table 6: Predictive factors of portal recanalization according to treatment in univariate analysis.
| Parameters | G1 (n=25) | p |
|---|---|---|
| The initiation of anticoagulant therapy | 13 | NS |
| Acute PTV | 9 | NS |
| Partial PTV | 9 | NS |
| Extensive PTV | 13 | p=0,009 |
Note: Group 1: Patients treated; Group 2: Untreated patients; PTV: Portal vein thrombosis.
Table 7: Predictive factors of portal recanalization according to treatment in multivariate analysis.
We found that anticoagulant therapy did not increase the risk of bleeding (p=0.686) or the risk of developing other complications. In our study, the mean survival was better in the patients treated successfully (38.31 months versus 23.41 months) without reaching a statistically significant difference (p=0.204) (Figure 1).
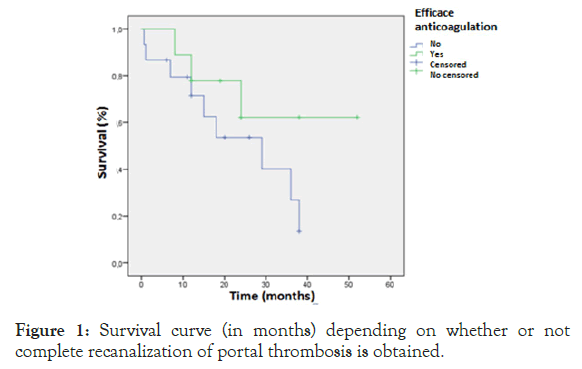
Figure 1: Survival curve (in months) depending on whether or not complete recanalization of portal thrombosis is obtained.
In recent years, clinical and laboratory studies have changed our perception of coagulation and hemostasis abnormalities associated with cirrhosis [3,10]. We have thus moved from the idea of a condition with a major risk of bleeding due to lack of coagulation, to that of a condition with an increased risk of thrombosis due to excessive coagulation [3,4].
In the literature, cirrhosis represents 22 to 28% of the causes of PVT in adults [6]. The results of epidemiological studies concerning the prevalence of PVT in cirrhotic patients are highly variable, depending in particular on the population studied. Analysis of cross-sectional studies with the largest numbers of cirrhotic patients shows an overall prevalence of extrahepatic PVT of 10 to 15% [6,7].
The prevalence of PVT in cirrhotic patients varies depending on the diagnostic means used. The current use of imaging techniques, especially ultrasound, allows detection of asymptomatic PVT, which is reflected in the gradual increase in prevalence in recent years [6].
The possible etiological factors of the constitution of the PVT remain obscure because it is very difficult to differentiate, by the available cross-sectional studies, what is cause or consequence [3,4].
Some authors have demonstrated a greater frequency of hereditary prothrombotic conditions in patients with PVT, except for the factor V Leiden mutation. These genetic mutations could therefore constitute additional factors of thrombosis in cirrhotic patients [8]. However, these data should be taken with some reservations because the prevalence of biological risk factors for PVT (innate or acquired) in cirrhotic patients varies considerably from one study to another [1,9]. However, it does not appear to be greater than that observed in the general population. Systematic screening for prothrombotic abnormalities cannot be recommended in cirrhotic patients with PVT [1].
While the PVT has long been considered a pejorative element in the natural history of cirrhosis, the most recent studies suggest that PVT does not increase the risk of decompensation and has no influence on the survival [11–13].
The aggravating role of PVT on the complications of cirrhosis is debated, because clinical studies are usually based on small cohorts of patients and short follow-up periods [12].
In a recent meta-analysis involving 2436 cirrhotic patients, PVT was associated with an increased risk of ascites decompensation, but the effect of PVT on gastrointestinal bleeding and hepatic encephalopathy was not evaluated due to insufficient data [14]. Contrary to these data, a multicenter prospective study in the same field, did not find a significant association between PVT and hepatic decompensation [13]. It is considered that the small number of studies evaluated and the lack of randomized controlled trials limit the generalization of findings of meta-analyzes [12].
To assess the prognosis of cirrhotic patients with PVT, a study published in 2015 was conducted by Berry et al. [15] on 66506 cirrhotic patients awaiting liver transplantation. PVT had occurred in 2207 patients. During follow-up, 27% of patients died before liver transplantation and 44% were transplanted. The death rate in patients without PVT was higher than in those with PVT. In the work of Nery et al., PVT was not associated with a high risk of mortality [13]. In our study, 16 patients or 32.65% of the total population died during follow-up.
The present study confirms that anticoagulation therapy in cirrhotic patients with non-neoplastic PVT is not associated with increased risk of liver disease decompensation, including bleeding. Although it is monocentric and retrospective, our work has made it possible to study the epidemiological, diagnostic, therapeutic and evolutionary profile of cirrhotic patients with PVT. The diagnosis of PVT is now made easier by the development of imaging techniques. Nevertheless, this condition deserves deep attention because of the complexity of its management. It remains a challenge for the clinician in Tunisia faced with the unavailability of adequate interventional therapeutic means. Interventional radiology techniques would improve the prognosis of PVT in cirrhotic patients. However, national multicenter studies involving a larger number of patients could better deduct prognostic factors.
Citation: Bizid S, Jlassi H, Abbes MB, Mohamed G, Ghedira H, Abdallah HB, et al. (2021) Risk Factors and Effects of Portal Vein Thrombosis on the Outcomes of Liver Cirrhosis in a Tunisian Cirrhotic Population. J Hepatol Gastroint Dis. 7:188.
Received: 16-Aug-2021 Accepted: 30-Aug-2021 Published: 06-Sep-2021 , DOI: 10.35248/2475-3181.21.7.188
Copyright: © 2021 Bizid S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.