Autism-Open Access
Open Access
ISSN: 2165-7890
ISSN: 2165-7890
Review Article - (2024)Volume 14, Issue 2
About 1/3 of autistic children experienced Developmental Regression (DR), a special subtype of Autism Spectrum Disorder (ASD) governed by heredity, environment and their interaction. With in-depth study of gut microbiota, a close association between the imbalance of gut microbiota in early life and the incidence of ASD in infants has been established. Brain development and gut microbial stability occur almost at the age of 2 to 3 years old, that is, the age at which DR occurs. Therefore, the manner in which gut microbiota imbalance affects DR in autistic children is currently a research hotspot, given that the close association between gut microbiota and ASD in infants has been established. This review firstly summarizes the mechanisms involved in the brain processes of autistic children with DR, including gene regulation, Mitochondrial Dysfunction (MD), oxidative stress and immune inflammatory reaction. Secondly, it focuses on gut microbiota’s role in it so as to provide reference for early clinical identification and intervention in autistic children with DR.
Autism; Gene regulation; Mitochondrial dysfunction; Oxidative stress; Inflammation; Gut microbiota
Autism Spectrum Disorder (ASD) is a complicated and challenging neurodevelopmental condition in children that is typified by an insufficient ability to communicate and interact socially, in addition to the manifestation of recurring and restrictive behaviors [1]. In a report by the Centers for Disease Control and Prevention (CDCP), 1/36 8-year-old children in 2020 was presume to have ASD and the incidence rate was rapidly increasing [2,3]. Genetics is fundamental to the pathogenesis of autism; however, many studies have shown that more than 50% of neurobiology in ASD is driven by non-genetic factors (such as advanced parental age, pregnancy infection and prenatal medication), in which there is a very strong gene-environment interaction [4,5]. Because ASD is a heterogeneous disorder, individuals with the disease usually present with a variety of associated conditions, including Gastrointestinal (GI) problems and Developmental Regression (DR) and autistic children with complications tend to have more severe cognitive impairment, which increases the difficulty of clinical management and increases the burden on families and society [6-8]. Therefore, in view of this heterogeneity, studies on the etiology of ASD are increasingly focused on subgroups of ASD patients, such as children with DR and GI symptoms [9].
As a form of ASD onset, DR describes a pattern in which a child experienced normal or near-normal growth followed by a form of growth plateau or the loss of previously acquired skills, which sees 30% or more of autistic children [10,11]. DR can be a presenting feature that associated with early ASD diagnosis and this has attracted increased attention [12-14]. DR in ASD is typically considered to occur between the first 15 and 30 months of an infant’s life, with a mean period of 21 months. At present, DR is divided mainly into language regression, language/social regression, motor regression and others; loss of language and social skill-related traits see far more research and review [15,16]. A great deal of evidence shows that autistic children with DR are more functionally impaired vis-a-vis language development, intellectual function and the severity of autism, with worse prognoses than autistic children without DR [17-19]. However, the clear cause of DR has yet to be determined clinically and genetic or immunological factors could be impactful on the development of DR [20]. Neurodegeneration underlies neurological function loss in autistic children with DR, including Mitochondrial Dysfunction (MD), oxidative stress, proinflammatory cytokines, activated microglia and astrocytes [21]. Given that autistic children with DR have more GI symptoms and their gut microbiota characteristics differ from those of autistic children without DR, gut microbiota’s involvement in the development of DR is increasingly worth exploring [22-24].
Studies have shown that autistic children have more than four times the GI problems that normal children have, with constipation, diarrhoea and abdominal pain being the most common issues [25]. GI dysfunction is also associated with the severity of ASD [26]. Particularly, autistic children with language regression were found to show more symptoms of GI problems and were from families with extensive celiac disease and inflammatory bowel disease records. Growing evidence has shown a link between alterations in gut microbiota composition and GI and neurobehavioral symptoms in autistic children [27]. Several investigations have established a relationship between gut microbiota and ASD using the brain-gut axis hypothesis, which links the gut to the Central Nervous System (CNS) through the immune system [28-31]. Reportedly, gut microbiota differs significantly between autistic children, especially children with DR and normal children. Autistic children had markedly elevated levels of Actinobacteria, Proteobacteria, Erysipelotrichi and Gammaproteobacteria, with Proteobacteria particularly amplified solely in autistic children with DR [32]. Finegold, et al., [23] observed that Desulfovibrio plays a key role in regressive autism by damaging the immune system and destroying intestinal tissues. However, the relationship between these abnormalities and the pathogenesis of DR in ASD are not clear. Deciphering how gut microbiota take part in the pathogenesis of DR is of great significance to identifying children at risk of ASD and formulating early intervention strategies and individualized treatment.
An imbalance in gut microbiota can affect the function of mitochondria, lead to oxidative stress and inflammation and impact nerve development, a crucial actor in DR pathogenesis; however, the specific mechanism of action here remains to be fully elucidated [33,34]. This review summarizes the exact mechanism through which gut microbiota influence the DR of ASD to provide reference for early identification of and intervention against ASD in children.
Pathological mechanisms of DR in ASD
Because a significant number of autistic children have DR, hypotheses that it is a possible neurobiological subtype with distinct potential factors of genetics and the environment abound [35]. Firstly, investigations on genetic syndromes, such as Rett syndrome, Methyl-CpG binding Protein 2 (MECP2) duplication syndrome, Phelan-McDermid syndrome or Landau-Kleffner syndrome, have reported relatively high incidences of regression [36-40]. Secondly, there are indications that environmental factors could account for regression under high genetic risk conditions; for example, parents often report DR after infection, vaccination, trauma and exposure to toxins or stress [41-45]. To explore the factors causing ASD with DR, the sharing mechanisms involved in the brain processes of autistic children and DR include gene regulation, MD, oxidative stress and immune inflammatory reaction were summarized as follows.
Gene regulation of ASD and DR: A comprehensive review of the degeneration of carriers of gene mutations closely associated with ASD identified phenotypes of regression in 7 genes (MECP2, SHANK3, CHD2, CHD8, CNTNAP2, KMT5B and SYNGAP1), which bore higher regression phenotypes than the 32% estimated in ASD [46]. To further elucidate the possible mechanisms by which these genes lead to ASD with DR, we summarized the functions, pathological mechanisms and Online Mendelian Inheritance in Man (OMIM) phenotype of these genes (Table 1) [47-68].
| Gene | Full name | Protein function | Pathological mechanisms | OMIM phenotype | Reference |
|---|---|---|---|---|---|
| MECP2 | Methyl-CpG binding protein 2 | Represses transcription from methylated gene promoters; regulates the development and function of neurons, astrocytes, oligodendrocytes and glial cells and synaptic plasticity and participates in alternative splicing. | Altered transcriptional landscape of astrocytes and disrupts energy metabolism and mitochondrial function. Decreased neuron size and reduced spontaneous or miniature Excitatory Post-Synaptic Current (mEPSC) frequency. Led to impaired mTOR signaling. | Autism, Rett syndrome (RTT), severe neonatal-onset encephalopathy with microcephaly, X-linked intellectual disability. | [47,48] |
| SHANK3 | SH3 and multiple snkyrin repeat domains 3 | Regulates splicing and protein structure, synapse establishment and synapse structure and function. | Impaired expression of several clustered protocadherins. Altered molecular composition of excitatory synapses and altered neurotransmission. | Phelan-McDermid syndrome, schizophrenia. | [49-51] |
| CHD2 | Chromodomain helicase DNA binding protein 2 | Modifies chromatin structure thus altering access of the transcriptional apparatus to its chromosomal DNA template; regulates human cortical interneuron development. | Led to deficits in neuron proliferation and a shift in neuronal excitability. Led to a prominent reduction of GABA. Led to an increase in the expression level of the REST gene. | Developmental and epileptic encephalopathy. | [52-55] |
| CHD8 | Chromodomain helicase DNA binding protein 8 | Functions in transcriptional regulation, epigenetic remodeling, promotion of cell proliferation and regulation of RNA synthesis. | Influenced oligodendrocyte maturation and transcription of neurogenesis, synaptic processes and neuroimmune signal transduction. Accelerated generation of inhibitory neurons and delayed generation of excitatory neurons. | Autism, susceptibility. | [56-58] |
| CNTNAP2 | Contactin associated protein 2 | Functions in nervous system as cell adhesion molecules and receptors; regulates Ca2+ homeostasis and neuronal network synchrony. | Neuronal migration abnormalities, reduced number of interneurons and abnormal neuronal network activity. Reduced excitatory synaptic transmission and balance of Excitation and Inhibition (E/I). Increased permeability of the Blood-Brain Barrier (BBB) and oxidative stress. | Autism, susceptibility, cortical dysplasia-focal epilepsy syndrome. | [59-62] |
| KMT5B | Lysine methyltransferase 5B | Involved in chromatin modification, occupation and gene regulation. | Induced synaptic dysfunction via alterations of DNA repair and gene transcription. Impaired dendrite development and led to decreased proliferation of neural progenitor cells and accelerated neuronal migration. | Intellectual disability, autosomal dominant. | [63,64] |
| SYNGAP1 | Synaptic Ras GTPase activating protein 1 | Controls dendritic maturation, synaptic function and network activity in developing human neurons. | Enhanced ERK and GSK3 phosphorylation. Impaired hippocampal synaptic plasticity. Disrupted neuronal E/I balance. | Intellectual disability. | [65-68] |
Note: OMIM: Online Mendelian Inheritance in Man; mTOR: mammalian Target of Rapamycin; DNA: Deoxyribonucleic Acid; GABA: Gamma-Aminobutyric Acid; RNA: Ribonucleic Acid; GTP: Guanosine Triphosphate; ERK; Extracellular signal-Regulated Kinase; GSK3: Glycogen Synthase Kinase 3.
Table 1: Phenotype and mechanism of genes with autism spectrum disorder and developmental regression.
The specific process by which mutations in ASD and DR risk genes converge on the neurobiology of the development and function of neurons and glial cells and synaptic formation, adhesion and transmission. It is worth noting that MECP2, SHANK3, CNTNAP2 and KMT5B are closely associated with MD; MECP2, SHANK3 and CHD8 have impact on oxidative stress; MECP2, SHANK3 and CHD8 are involved in immune inflammatory reaction. Although SYNGAP1 is not involved in MD, oxidative stress and immune inflammatory reaction, it clearly induces cognitive impairment in ASD by affecting the maturation of dendritic spines, glutamate receptor transport and synaptic function [69,70]. Above all, gut microbiota and its metabolites have been shown to regulate these processes.
Therefore, we speculate that MD, oxidative stress and immune inflammatory reaction may be the key mechanisms for communicating genetic mutations and intestinal microbiota abnormalities in ASD with DR.
Mitochondrial Dysfunction (MD): Mitochondria is pivotal to producing Adenosine Triphosphate (ATP), Reactive Oxygen Species (ROS) and active in immune response, modulating the levels of intracellular calcium ions and up keeping gut microbiota [71]. MD includes impairment of Oxidative Phosphorylation (OXPHOS) and metabolic, accumulation of unfolded proteins, loss of membrane potential, increased production of ROS, etc., [72].
Mitochondrial involvement is highlighted in the study of the pathogenesis of ASD, with meta-analysis has indicated that 30%-50% of autistic patients showed biomarkers of MD [73,74]. Remarkably, DR were observed in 52% of autistic children with MD, a more significant value than that in the overall autistic population. Shoffner, et al., [75] showed that autistic children with mitochondrial disease developed autistic symptoms after sudden rapid DR concerned in fever. Particularly, the respiratory function and glycolysis of mitochondria in autistic children with DR were more severely damaged than those in autistic children without DR. Autistic children with DR expressed a unique metabolic phenotype and their mitochondria were in a fragile state, which allowed mitochondrial decompensation under physiological stress, leading to clinical DR [76]. Evidence was found that MD leads to reduced release of synaptic neurotransmitters, such as inhibitory Gamma-Aminobutyric Acid (GABA)ergic interneurons, which performed a vital role in brain development in children aged 12-30 months, the most commonly reported age range for DR in ASD [77,78].
(a): Mitochondrial dysfunction and calcium regulation: A quantity of energy is needed to the maintenance of ion transport and synaptic transmission during neuronal signaling, a process that requires the essential participation of mitochondria. Changes in Ca2+ levels have been noted in brains of autistic children, implying that the subsequent impairment of the function of mitochondria can give rise to neuronal defects in synapse activity and damage neurotransmitters, as well as neuron migration and plasticity. The homeostasis of Ca2+ within cells modulates the discharge of excitatory neurotransmitter glutamate [79]. MD can cause a decrease in the discharge of synaptic neurotransmitters in neurons with elevated release rates, like restrictive GABA intermediate neurons [80,81]. Hence, MD can result in diminished inhibition and a comparative rise in the Excitation/Inhibition (E/I) ratio of ASD. Furthermore, an increment in the levels of Ca2+ within cells facilitates MD, which harms OXPHOS and generates oxidative stress. Damaged calcium signaling probably interferes with proliferation, migration, purkinje cell development, synaptic formation and maintenance involving in the link between MD and DR [82].
(b): Defects in the regulators of the biogenesis of mitochondria: Adenosine Monophosphate-activated Protein Kinase (AMPK) has been established as a key modulator of the metabolism of mitochondria in neurons [83,84]. Protein Kinase A (PKA) activates Cyclic-Adenosine Monophosphate (AMP) Response Binding Protein (CREB), which promotes downstream gene transcription and protein synthesis, contributing to long-term memory formation [85-87].
A decline in PKA activity during regressive autism could bring about a decrease in CREB phosphorylation and defects in cellular signaling. Additionally, AMPK obstructs the mammalian Target of Rapamycin (mTOR) pathway, which is essential for regulating learning and memory processes [88]. Autopsy reports of autistic patients have shown that the increased density of excitatory synapses in the brain is related to abnormal mTOR-dependent synaptic pruning [89].
(c): Mitochondrial dysfunction and gene regulation: It includes MECP2, SHANK3, CNTNAP2 and KMT5B genes.
MECP2 mutations greatly affect the brain bioenergy regulation that may be mediated by MD [90]. Altered mitochondrial str ucture and deficiencies in mitochondrial enzyme activity were highlighted in different cells or tissues derived from Rett (RTT) animal models and patient specimens [91]. Cicaloni, et al., [92] revealed an altered expression of mitochondrial fusion and mitophagy genes along with the evidence of aberrant mitochondrial dynamics and mitophagy in RTT cells in a large-scale proteomic analysis.
Regarding SHANK3, systems biology has revealed that 63 mitochondrial proteins in the cortex of SHANK3 mutation mouse model were S-nitridation and several mitochondria-related processes are enriched, including respiratory electron transport chain, stress response, regulation of neuron apoptotic process and ROS metabolic process [93]. Wang, et al., [94] reported that SHANK3 can directly bind to Calcium-Calmodulin (CaM)-dependent Protein Kinase II (CaMKII) and block the mitochondrial translocation of CaMKII, resulting in the inhibition of mitochondrial autophagy, which ultimately leads to MD.
Protein CASPR2 (coded by CNTNAP2), a member of the neurexin family, plays a crucial role in the balance of excitatory and inhibitory post-synaptic currents [95]. An integrative multi-omics analysis of quantitative proteomic data from CNTNAP2 knockout mice, ASD patients and forebrain organoids revealed systemic changes in mitochondria (glycolysis processes, lipid metabolism and mitochondrial energy production) [96].
Mice lacking KMT5B proteins activate Peroxisome Proliferator-Activated Receptor gamma (PPAR-γ) target genes in brown adipose tissue to increase mitochondria respiration, improve glucose tolerance and reduce adipose tissue to fight obesity, which suggests that the role of KMT5B in neural development may be related to mitochondria function [97].
Oxidative stress
Oxidative stress is a pathologic state effected by the imbalance between free radicals, like ROS and cell detoxification ability; this maladjustment brings about severe injury to all macromolecules (protein, lipid and Deoxyribonucleic Acid (DNA)) and disturbs many signaling pathways [98]. Compared with other organs, the tolerance of the brain to oxidative damage is relatively weak, so oxidative stress in the pathological process of mental illness has always attracted much attention [99]. Some reported a significant increase in DNA oxidation in the frontal cortex, temporal cortex and cerebellum in individuals with ASD compared to controls [100,101]. Reportedly, autistic children are more vulnerable to oxidative stress because they have low levels of plasma and cellular free Glutathione (GSH), as well as reduced storage and antioxidant capacities, which could set in motion epigenetic change and neurodevelopmental disorders, inflammation of neurons, damage to the brain and neurological disorders [102,103]. Some found that the density of lipofuscin, a result of oxidative injury in the tissues, was observed to be greater in cortical brain areas concerned with communication in autistic children than in controls. As mentioned earlier, loss of speech and language is the most common form of DR.
Oxidative stress and mitochondrial dysfunction: A link has been established between the impairment of mitochondria and oxidative stress in tissues of the brain collected from autistic children. Oxidative stress leads to MD and dysfunctional mitochondria produce superfluous mitochondrial ROS (mROS) [104,105]. Frye, et al., [106] findings, mitochondrial disease-suffering ASD children had lower amounts of free GSH. Analyses of lymphoblastoid cell lines collected from some autistic children bore heightened oxidative stress, reduced GSH redox capacity and extremely active mitochondria with enhanced susceptibility to ROS [107]. In addition, excessive ROS produced by mitochondria leads to the imbalance of redox in Rett (RTT) syndrome, which plays an important role in DR [108]. Moreover, complex I and complex II-dependent overproduction of H2O2 was proved in mitochondria separated from the brains of MECP2 knock-out mice [109,110].
Oxidative stress and gene regulation: It includes MECP2, SHANK3 and CHD8 genes.
(a): MECP2: Oxidative stress is conducive to the pathogenesis and severity of RTT and may be a crucial auxiliary factor in the mechanisms of DR. The most convincing evidence is the significantly improved phenotype and reduced levels of brain oxidative stress markers in MECP2 mutant mice following MECP2 re-expression [111]. Grosser, et al., [112] detected an increased Flavin Adenine Dinucleotide (FAD)/Nicotinamide Adenine Dinucleotide Hydrogen (NADH) baseline-ratio indicating intensified oxidization in hippocampal slices of a Rett mouse model. Pintaudi, et al., [113] found increased lipid peroxidation and imbalance of metallothionein expression in patients with RTT by evaluating blood oxidative stress levels.
(b): SHANK3: Amal, et al., [114] reported that the concentrations of 3-nitrotyrosine and S-nitrosoglutathione in SHANK3 mutants increased significantly, sending signals of oxidation and nitroso stress. SNO-calcineurin significantly increased phosphorylated synapsin1 and CREB, affecting synaptic vesicle mobilization and gene transcription, respectively. And pathway analysis showed that the processes affected in ASD were enriched.
(c): CHD8: CHD8 is an ATP-dependent protein that represses transcription by altering nucleosome positioning and regulates a network of genes critical for early neurodevelopment [115]. Modafferi, et al., [116] observed higher levels of ROS and lower level of GABA neurotransmission in CHD8+/- than in CHD8+/+ spheroids.
Immune inflammatory reaction
Persistent inflammation is the principal cause of the pathogenesis/ progression of ASD and is also considered to be the basis of DR [117,118]. The earliest indication of the connection between ASD and inflammation is the manifestation of heightened ASD occurrence in children whose mothers have a history of infection in the course of pregnancy [119]. Moreover, a substantial number of parents with autistics children have reported incidents of otitis managed with oral broad-spectrum antibiotics before the expression of symptoms of regressive autism in children [120]. Reports have shown that immunotherapy, like corticosteroids, contributed to the recovery of regressive autism [121,122]. A study found that members of families with children suffering from regressive autism had considerably more cases of autoimmune diseases relative to autistic children without DR [123]. Hacohen, et al., [124] examined two children with regressive autism, eventually diagnosing them with anti-N-Methyl-D-aspartate (anti-NMDA) receptor encephalitis. Immune impairments, like inflammatory and autoimmune processes are reportedly autism events, especially in regressive forms include enhanced proinflammatory marker levels and microglial activation [125,126]. In a nutshell, these reports appear to implicate inflammatory disorders in the causation of the clinical subset of interest here at specific times.
(a): Changed pro-inflammatory cytokines and adhesion molecules level: Investigations have shown differences in a variety of cytokines and Nerve Cell Adhesion Molecules (NCAM) between autistic children with DR and without DR [127-129]. Reportedly, the levels of Interleukin (IL) 1β, IL-5, IL-17, TNF-α as well as total Th2 and Th17 cytokines were higher in autistic children with DR [130-132]. Among them, the level of IL-1β has been considered to be positively correlated with the severity of ASD. It can cross the Blood Brain Barrier (BBB) and affect all aspects of the development and function of neurons and glial cells [133]. IL-5, produced by Th2 cells and mast cells, stimulates B-cell growth and increase immunoglobulin secretion. The presence of maternal brain protein-specific autoantibodies has also been shown to be closely associated with regressive autism. TNF-α is involved in pro-inflammatory signal and has been shown to infiltrate the BBB, leading to cognitive and behavioral defects in autistic children [134]. Th17 cells normally function by secreting IL-17, which exerts a pro-inflammatory effect and activate CNS microglia, thereby heightening the presence of a range of factors, including CNS transforming growth factor-β (TGF-β) [135,136]. Treg cells and Th17 cells play the opposite role in immune response and their imbalance has been proved to influence the pathogenesis of ASD [137]. In addition, NCAM1 was higher in children with DR ASD than in children without DR; while the level of NGF was lower. Both of them play a key role in the development of the nervous system and synaptic plasticity pathways.
(b): Activation of microglia: Microglia reside in the CNS as macrophages and exist predominantly in a resting state in standard physiological environments, during which they continually monitor their microenvironment, modulate synaptic pruning, a major step in the promotion of the formation of synapses and neuronal activity and synaptic plasticity regulation, via pathways, like complement and CX3CR1 signal transduction [138-145]. Dong, et al., [146] reported that Negative Regulator of Reactive Oxygen Species (NRROS) mutation damage led to uncontrolled microglia activation and neuroinflammation leading to infant DR. The activation of microglia, which sees their numbers inflate, occurs in reaction to the dysregulation of mitochondria, oxidative stress or neuroinflammation [147-149]. Once activated, microglia transform and become amoeba-like before migrating to sites of inflammation and secreting proteins, including cytokines, chemokines and ROS, which are possible triggers of synaptic plasticity and learning and memory deficiencies linked to autism. In the hippocampus, dendritic spines multiply and the microglial receptor (CX3CR1) levels diminish, lessening microglia motility, which leads to reduced contact between microglia and synapses [150]. Synapse numbers in RTT patients and mice have also been shown to change, with several basic pathologies in Rett syndrome mice rescued after the transplantation of wild-type microglia [151,152].
(c): Immune inflammatory reaction and mitochondrial dysfunction: As the hub of OXPHOS and many other metabolic processes, mitochondria are essential to forming and sustaining an efficient immune system. An impairment of mitochondria would inevitably impact recognition of pattern recognition receptors and trigger inflammation. In Napoli, et al., [153] research, deficits in immune response in granulocytes collected from ASD children with impaired mitochondria were amplified 1.6-fold. These impaired mitochondria are the principal activators of regions of Nucleotide-binding Domain, Leucine-Rich, Pyrin-3 (NLRP-3) inflammatory bodies that are rich in leucine repeats, which are responsible for Caspase-1 activation. An activated Caspase-1 subsequently splits and stimulates cytokines IL-1β and IL-18, fostering pathways of inflammation and self-defense. In addition, mROS have gradually gained recognition as activator of NLRP3 and critical regulators of Nuclear Factor (NF)-κB, Mitogen-Activated Protein Kinases (MAPKs) and Interferon Regulatory Factor (IRF) signaling, given that they impact both innate and adaptive immune responses [154,155].
(d): Oxidative stress and immune inflammatory response: The link between oxidative stress and inflammation is well document. The accumulation of oxidized proteins leads to the secretion of inflammatory signals and stimulate macrophages to produce TNF-α, essential to regulate synaptic strength and plasticity [156,157]. High levels of ROS activate various signaling pathways that produce pro-inflammatory chemicals and create a vicious circle to maintain high secretion of pro-inflammatory cytokines and chemokines, leading to neuroinflammation and neuronal degeneration in autism [158]. In addition, lower GSH levels can also promote inflammation [159]. GSH exerts vital antioxidant and neuromodulation functions in the brain and fosters neuronal survival. As a neuromodulator of glutamate ionotropic receptors, GSH interacts with the receptors of NMDA and guards against the excitotoxicity of glutamate [160]. The regulation of GSH is also related to the regulation of redox sensitive transcription factor NF-κB and Activator Protein 1 (AP-1), as well as downstream signal transduction (pro-inflammatory cytokines and inducible enzymes), which has a significant effect on neuroinflammation. Moreover, a depletion in GSH levels would cause oxidative damage in the brain, leading to neurodegenerative changes [161].
(e): Microglia and gene regulation: It includes MECP2, SHANK3 and CHD8 genes.
(i): MECP2: Microglia from MECP2-null mice proved to participate in the release of abnormally high levels of glutamate, which has neurotoxic effects on hippocampal neurons, including dendritic and synaptic destruction through CX3CR1 pathway and glutamine transporter SNAT1 overexpression [162]. Overexpression of SNAT1 in MECP2-deficient microglia would destroy the glutamine homeostasis, leading to MD and microglial neurotoxicity caused by excessive glutamate production [163].
(ii): SHANK3: Knockdown SHANK3 expression in neuronal cultures specifically reduced the synaptic expression of the metabotropic Glutamate Receptor 5 (mGluR5) [164]. Chana, et al., [165] demonstrated that in mGluR5 Knockout (KO) mice there is a significant increase in microglial density, which is of relevance to characteristics seen in individuals with ASD.
(iii): CHD8: In vivo, CHD8 co-localized in neurons, astrocytes and microglia [166]. Silencing CHD8 in microglia altered insulin-like growth factor-1 messenger Ribonucleic Acid (mRNA) expression following lipopolysaccharide stimulation and prevented the differentiation of oligodendrocyte precursor cells to oligodendrocytes [167].
Mechanism of gut microbiota in Autism Spectrum Disorder (ASD) and Developmental Regression (DR)
Gut microbiota is a group of microorganisms that live in the GI system of the human body. This group of microorganisms participates in the regulation of physiological functions, such as digestion, absorption, excretion, immunity, synthesis and decomposition of amino acids, bile acid metabolism and vitamin synthesis [168]. In recent years, with the in-depth study of gut microflora and the CNS, gut microbiota is considered to be the key medium of the CNS and intestinal system [169]. The frequent combination of GI symptoms in autistic children raises the hypothesized association between ASD and gut microflora. In particular, children with DR were more likely to have diarrhea/loose stools and vomiting; autistic children and GI symptoms showed a higher percentage of DR than those without GI symptoms, suggesting a connection between gut microbiota and DR [170,171].
One investigating team considered a toxin-producing Clostridium as the cause of DR because of the establishment of neurobehavioral symptoms and chronic diarrhea in a group of autistic children with DR after repeated courses of antibiotics led to consider a toxin-producing Clostridium as the cause of DR. In follow-up studies, Sandler, et al., [172] further supported this hypothesis. The children with regressive autism received oral vancomycin, an anti-Clostridium drug, for 6 weeks and this drastically improved the neurobehavioral symptoms and GI symptoms of 8/10 children. In addition, gut microflora in children with DR ASD expressed more Desulfovibrio, less Bifidobacterium and Akkermansia muciniphila (a species that might predispose to bacterial translocation and promote gut dysfunction) than those without DR [173]. Therefore, we reviewed the mechanism of gut microbiota and related metabolite influencing brain and behavior, including the function of mitochondria, the activation of oxidative stress, immune inflammatory response and the interaction with host genetics, which are correspond to the pathogenesis of DR.
(a): Gut microbiota and mitochondrial dysfunction: The incidences of DR (52%) and GI issues (74%) are more frequent in mitochondrial disease-ridden autistic children relative to the overall population of autistic children. Microbiota protects the structural and functional integrity of mitochondria and specific microbial products may inhibit or fine-tune the function of mitochondria [174-176]. Because mitochondria are the main targets of organic poisons, when the gut microbial detoxification of autistic children is seriously damaged, more toxic substances from external and internal sources may enter the circulation and damage the mitochondria of various tissues, making them vulnerable to environmental toxins. In addition, the increase of mitochondrial Electron Transport Chain (ETC) complex protein content was mainly seen in the cecum, where the gut microbiota gut microbiota ferments, which indicates that gut microbiota was crucial in the evolution of MD in autistic children [177]. Clostridium-produced Single-Chain Fatty Acids (SCFAs) can enter the mitochondria and be used as substrates to generate energy. This process enhances the production of NADH and lead to the increase of ETC complex I expression. Remarkably, children with impairment to their mitochondria also harbor GI issues identical to those in children with autism, pointing once more to the correlation between mitochondrial damage, GI complications and the microflora of patients with autism [178].
(b): Gut microbiota and oxidative stress: The gut microbiota can significantly affect systemic antioxidant status [179]. SCFAs from gut microbiota can directly influence mitochondria’ function by changing its membrane composition, thus changing the tricarboxylic acid cycle, leading to oxidative stress and reducing the production of GSH [180-182]. The production of SCFAs and secondary bile acids by intestinal bacteria increases the release of ROS from intestinal epithelial cells, which leads to TNF-α-induced inflammation and the activation of many cytokines. One study found that the presence or absence of a microbiota alters gene expression in host GI tissues. Specifically, gut microbiota has a profound systemic effect on amino acids and GSH as well as lipid metabolism and it is one of the major regulators of metabolism in mammals [183]. However, the microbiota of ASD lacks the biosynthesis of GSH, so individuals are more vulnerable to oxidative stress.
(c): Gut microbiota and brain inflammation: The gut microbiota and its metabolites are the chemical and biological barriers of the intestinal mucosa and are involved in immune system development, as well as its regulation, especially in years one to three of life. The inadequacies of immune system create a period during which infants are particularly vulnerable to infections which, if treated with broad-spectrum antibiotics especially, can cause gut dysbiosis and GI disorders. Inflammatory intestinal pathology has been reported in children with regressive autism [184]. The ecological disorder of gut microbiota in autistic children is thought to be related to intestinal epithelial inflammation, intestinal blood barrier and increased BBB permeability, which leads to inflammation of the central nervous system.
(i): Peripheral immune system activation: Gut microbiota, particularly during early life severely affect peripheral immune system growth, maturation and activation [185,186]. Several investigations have reported a link between ASD and GI barrier defects, commonly known as leakage gut, as well as between ASD and compromised BBB integrity [187-189]. An imbalance in intestinal florae is a recipe for damage to the intestinal epithelial barrier and for increased permeability of the intestinal mucosa, which would facilitate the passage of a large number of toxins, bacteria and their constituents and metabolites, such as Lipopolysaccharide (LPS) and SCFAs, through the intestinal epithelial barrier, thereby activating the immune response and introducing an inflammatory state into the bloodstream. The increase of pro-inflammatory cytokines in plasma, like IL-1β, IL-17, TNF-α, is related to autism, especially regressive autism. During a systemic inflammatory response, inflammatory factors in the blood circulation can bind to the surfaces of intestinal mucosal epithelial cells and cerebrovascular endothelial cells, activate Toll-like Receptors (TLRs) and stimulate intestinal mucosal epithelial cells and cerebrovascular endothelial cells. Inflammatory responses in cells aggravate the damage to the intestinal mucosal barrier and BBB. Inflammatory factors breach the CNS via the impaired BBB and trigger an inflammatory response in the CNS. IL-1β and TNF-α can lash on to nerve cell surfaces, causing the hyper activation of microglia and neuroinflammatory responses, resulting in ASD manifestations [190-192].
(ii): Microbiota-microglia modulation: Microglia are extremely sensitive to environmental changes and are easily activated by systemic inflammation or circulating endotoxin, especially in areas of the CNS with windowed capillaries, like the choroid plexus [193,194]. Communication between microflora and microglia is mediated by a variety of mechanisms, which are either direct or indirect, like bacterial metabolite production, e.g., SCFAs, direct peripheral immune system and cytokine environment regulations and direct Vagus Nerve (VN) activation through the compounds and metabolites of bacteria [195-197]. In the course of homeostasis, various metabolites and immune system components from bacteria have the capability of triggering the VN or reaching the brain through systemic circulation, impacting microglial maturation and function directly. For example, propionate produced by Clostridium, a kind of SCFA, significantly increases the activation of microglia, which would amplify local inflammatory cytokine production, leading to bystander damage and the occurrence of ASD behavior. In addition, if intestinal permeability is high, caused by the imbalance of gut microbiota, bacteria may be translocated and an imbalance in circulating bacterial derivatives may arise, which would stimulate immune signaling pathways, including cytokine and other pro-inflammatory molecule release. Both these components of bacteria and pro-inflammatory facilitators can breach the BBB or stimulate the VN, generating abnormal microglia homeostasis, e.g., monitoring, synaptic pruning and inflammation, resulting in ASD symptoms (Figure 1) [198].
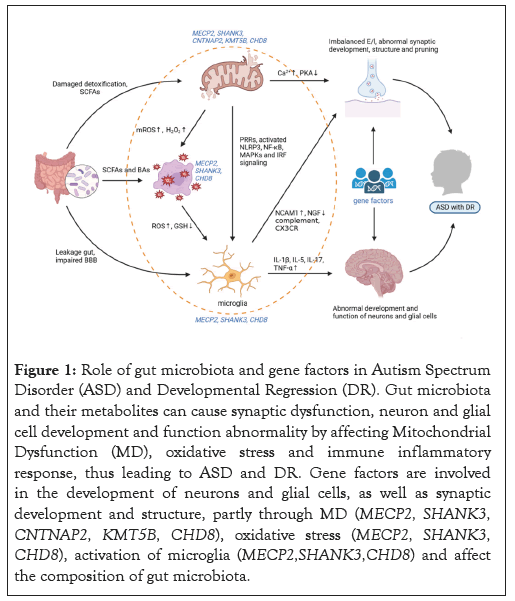
Figure 1: Role of gut microbiota and gene factors in Autism Spectrum Disorder (ASD) and Developmental Regression (DR). Gut microbiota and their metabolites can cause synaptic dysfunction, neuron and glial cell development and function abnormality by affecting Mitochondrial Dysfunction (MD), oxidative stress and immune inflammatory response, thus leading to ASD and DR. Gene factors are involved in the development of neurons and glial cells, as well as synaptic development and structure, partly through MD (MECP2, SHANK3, CNTNAP2, KMT5B, CHD8), oxidative stress (MECP2, SHANK3, CHD8), activation of microglia (MECP2,SHANK3,CHD8) and affect the composition of gut microbiota.
(d): Gene regulation and gut microbiota: The relationship between host genetics and microbiota composition is an important and understudied area of research. Recent studies have shown that the microbiota act as an important modulator of the gene-phenotype interactions [199]. Liu, et al., [200] proposed extent host Single Nucleotide Variants (SNVs) in ASD impact the functions of microbiota by altering microbial pathways and microbiome gene abundance. In addition, gut microbiota can regulate gene transcription, expression and protein abundance by regulating the level of RNAs or noncoding RNAs [201]. The gut microbiota has been shown to regulate the expression of miRNA in the amygdala and Prefrontal Cortex (PFC) of Germ-Free (GF) mice [202]. Therefore, we summarize the evidence of dysfunctional microbiota in the gene mutations highly related to DR, indicating that ASD and DR caused by these gene mutations may be achieved partly by altering gut microbiota.
The microbial richness of RTT subjects was lower than that of healthy controls and the dysfunctional microbiota produced altered short chain fatty acids profiles [203]. Sauer, et al., [204] reported expression in GI epithelium, a significantly different GI morphology and an altered microbiota composition of SHANK3 KO mice that may contribute to inflammatory responses affecting brain development. Changes to gut amino acid transporters and microbiota associated with increased E/I ratio in CHD8+/- mouse model of ASD-like behavior [205]. Buffington, et al., [206] discovered the social-behavior phenotype of CNTNAP2 mice is mediated by the gut microbiota and specific microbial intervention selectively rescued the social deficits in CNTNAP2 mice through upregulation of metabolites in the tetrahydrobiopterin synthesis pathway.
To sum up, we summarized the crosslinking mechanism of genetic, MD, oxidative stress and immune inflammatory reaction affecting the occurrence of DR in ASD and described the role of gut microbiota in it. By triggering MD, increasing ROS levels and aggravating neuro-inflammation, gut microbial and its metabolites can promote imbalanced E/I and abnormal synaptic development, thereby resulting in the occurrence of DR. Our review provides useful ideas for early identification and potential therapeutic targets of this part of ASD children. In the future, it is necessary to comprehensively study the relationship between specific DR typing and microbiota and the effectiveness of intestinal flora therapy in clinical practice, in order to provide comprehensive theoretical support for individualized and targeted treatment of gut microbiota to prevent/delay the development of ASD.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Cui J, Li H, Hu C, Wu D, Li H, Luo X, et al (2024) Role of Gut Microbiota in the Developmental Regression of Autism. Autism-Open Access. 14:399.
Received: 31-May-2024, Manuscript No. AUO-24-30227; Editor assigned: 03-Jun-2024, Pre QC No. AUO-24-30227 (PQ); Reviewed: 17-Jun-2024, QC No. AUO-24-30227; Revised: 24-Jun-2024, Manuscript No. AUO-24-30227 (R); Published: 02-Jul-2024 , DOI: 10.35248/2165-7890.24.14.399
Copyright: © 2024 Cui J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.