Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Original Research Article - (2022)Volume 12, Issue 1
Introduction: Community Acquired Pneumonia (CAP) is a leading infectious cause of death in developed countries. The role of statins in CAP is contentious and limited studies are available in the Australian healthcare-settings. This study determined whether statin-usage has any beneficial effects on clinical outcomes in hospitalized CAP patients.
Methods: All adult CAP patients admitted to a tertiary-care hospital over a period of 3-months were included in this study. Data regarding patients’ demographic characteristics, pneumonia-severity, statin-usage and clinical-outcomes were obtained from medical records. Propensity-score matching was used to match known confounders between statin-users and non-users. The primary outcome measure was to determine whether statin-users had a reduced in-hospital or 30-day mortality when compared to statin non-users. Secondary outcome measures included any differences in length of hospital-stay, intensive-care-unit admission and 30-day re-admissions between the two groups.
Results: This study included 140 patients with CAP, mean age 69.3 (SD 17.2) (range 21-97) years, 52.1% were females. Fifty-six (40%) patients were on-statins at the time of hospital admission. Statin-users were more likely to be older males, with a higher Charlson-index and cardiovascular risk factors than statin non-users (P<0.05). When compared to statin non-users, statin-users had higher pneumonia-severity scores but significantly lower CRP levels (P<0.05). There were no differences in in-hospital mortality (2(2.4%) vs. 2(3.6%), P>0.05) or 30-day mortality (6 (7.1%) vs. 5 (8.9%), P>0.05), between the two groups. Other clinical outcomes were also similar between the two groups (P>0.05).
Conclusion: This study suggests similar clinical outcomes for CAP between statin-users and non-users.
Community acquired pneumonia; Statins; Mortality; Inflammation; Pneumonia severity
ANCA: Anti-Neutrophil Cytoplasm Auto-antibodies; BNP: Brain Natriuretic Peptide; CAP: Community Acquired Pneumonia; CCI: Charlson Comorbidity Index; CRP: C-Reactive Protein; DRG: Diagnosis Related Group; FMC: Flinders Medical Centre; HMG-CoA: 3-Hydroxy-3-Methylglutaryl-Coenzyme A; ICU: Intensive Care Unit; IQR: Interquartile Range; IHD: Ischaemic Heart Disease; LOS: Length of hospital Stay; MET: Medical Emergency Response; PSI: Pneumonia Severity Index; SD: Standard Deviation; WHO: World Health Organisation
As per a fact sheet published by the WHO, lower respiratory tract infections (including pneumonia) remain the most lethal infectious disease worldwide, and were responsible for 3.0 million deaths in 2016 [1]. An Australian study spanning over a period of 3 years found that in comparison to other infectious diseases, Community Acquired Pneumonia (CAP) was associated with the highest inhospital mortality and was responsible for the highest number of Intensive Care Unit (ICU) admissions between 2011 and 2013 [2].
Statins competitively inhibit 3-Hydroxy-3-Methylglutaryl-Coenzyme A (HMG-CoA) reductase (the rate limiting enzyme in the mevalonate pathway) resulting in reduced cholesterol synthesis in the liver [3]. In addition to the reduction in cholesterol levels, statins display an array of pleiotropic properties, including anti-inflammatory and immunomodulatory effects [4]. The anti-inflammatory effects of statins are mediated through inhibition of the synthesis of isoprenoid intermediates in the mevalonate pathway. Cells utilise isoprenoids for the post-translational modification and activation of small guanosine triphosphatases (GTP-ases e.g., Ras and Rho) [5]. These GTP-ase molecules are essential for mediating a number of inflammatory processes including adhesion, cell recruitment, and immune cell de-granulation [5].
The anti-inflammatory and immunomodulatory effects of statins have generated interest in repurposing these drugs to harness benefits in patients with infection. Several experimental and clinical studies have indicated that statins attenuate pulmonary inflammation by modulating neutrophil function and reducing cytokine expression and release [6-10]. Several observational studies have demonstrated that statin users may have a survival benefit among patients with CAP [11-13]. However, these benefits have so far not been replicated in randomised controlled studies.
Very limited studies have determined the benefits of statins among patients with CAP in Australian health care settings. Therefore, we conducted a retrospective observational study to assess the role of statins on clinical outcomes among patients hospitalized with CAP. The hypothesis for this research was that statins users will have a lower in-hospital mortality and 30-day mortality when compared to statin non-users.
All adult patients presenting with CAP to Flinders Medical Centre (FMC) for admission between 1st January and 31st March 2018 were included in this study. FMC is a 600-bed tertiary teaching hospital and caters to a population of around 375,000 in the southern suburbs of Adelaide, South Australia. From the Electronic Medical Records, we generated a master list of all admitted patients who had a Diagnosis Related Group (DRG) code for CAP. The list was screened by a member of the research team to ensure that the patients included in the study had symptoms of respiratory tract infection (e.g., fever, dyspnoea, cough, and sputum production) on presentation and imaging evidence (chest x-ray or CT chest) of an infiltrate. Patients who were found to have a Healthcare Associated Pneumonia (HCAP) (including those from nursing homes) were excluded from this study.
Data were collected from both the EMR and review of the case notes. We extracted the following variables for this study: Age, gender, residential status, Length of Hospital Stay (LoHS), whether or not patients were on statins at the time of hospital admission, type of statin used, type of antibiotics used, whether patient had a Medical Emergency Response (MET) call during admission, admission to ICU, in-hospital mortality, 30-days mortality, and re-admission within 30-days of hospital discharge. We also recorded investigations performed during admission; white cell count, C-Reactive Protein (CRP), troponins, Brain Natriuretic Peptide (BNP), blood culture, sputum culture, respiratory viral Polymerase Chain Reaction (PCR), and chest x-ray. Vital signs at the time of hospital admission were recorded including pulse rate, blood pressure, respiratory rate,and arterial oxygen saturation (SaO2). The relevant co-morbidities were documented, and Charlson Comorbidity Index (CCI) was calculated [14]. The severity of pneumonia was assessed by using the CURB-65 score [15]. The CURB-65 score is based upon four bedsides and one laboratory based prognostic markers. A large multicenter derivation/validation study has shown that this tool has a sensitivity of 75% and a specificity of 75% in predicting death at 30 days among patients with CAP [16].
The ethical approval for this study was granted by the Southern Adelaide Human Clinical Research Ethics Committee (SA HREC) no 187.8 on 23/07/2018 and this study was registered with the Australia and New Zealand Clinical Trial Registry.
Outcome measures
The primary outcome measure for this study was to determine whether patients who were on statins at the time of hospital admission had a reduced in-hospital mortality or 30-day mortality when compared to statin non-users. Secondary outcome measures included any differences in LOS, admission to ICU, number of MET calls during index admission and re-admissions within one month following hospital discharge between the two groups.
Statistical analysis
Data were visually assessed for normality with the use of histograms. Continuous variables were compared with the use of the t-tests or the Wilcoxon rank-sum tests (a non-parametric test) depending upon the distribution of data, while categorical variables were compared with the use of chi squared statistics or Fisher’s exact test, where appropriate. LOS was adjusted for in-hospital mortality. Scatter plot with line fit was used to display distribution of CRP levels and CURB65 scores according to the statin usage. Logistic regression was used to determine whether in-hospital mortality, admission to the ICU, 30-day mortality and 30-day readmissions were any significantly different between statin users and nonusers, after adjustment for the following co-variates; age, sex, CCI and CURB65 score. Survival curves were plotted, and a log rank test compared probability of survival between the two groups. Multivariable linear regression was used to determine whether LOS or number of MET calls was different between the two groups after adjustment for the above mentioned co-variates. In addition, we used propensity score matching to compare different outcomes between statin users and non-users.
Propensity score methods
We generated propensity scores using a multi-variate logistic regression model based on various confounding variables, as identified by the univariate analyses [17]. The variables associated with the treatment allocation (use of statins) and the primary endpoints of mortality were selected for input into a logistic regression model to calculate propensity scores. We used a matching algorithm employing the nearest neighbour matching method in a 1:1 ratio and applied a caliper (maximum probability distance) width of 0.20 [18]. The variables used in the model were age, sex, Charlson index, and history of Ischaemic Heart Disease (IHD), stroke, creatinine levels and CURB65 score. In the matched cohort, we then compared following clinical outcomes; in-hospital mortality, 30-day mortality, whether admitted to ICU and 30-day readmission, by assessment of the average treatment effect according to statin use.
Between 1st January and 31st March 2018, 140 patients with CAP were admitted to FMC. The mean age was 69.3 (SD 17.2) years with a range of 21-97 years and 73 (52.1%) were females. Fifty-six (40%) patients were on statins at the time of hospital admission. Among patients who were on statins, atorvastatin was the most frequently prescribed statin in 22 (39.3%) patients, followed by rosuvastatin 20 (35.7%) and simvastatin 14 (25%). The mean (SD) dose of atorvastatin was 33.5 (18.7) mg, rosuvastatin 12.5 (6.1) mg and simvastatin 23.6 (11.5) mg. Patients who were statin users were more likely to be older males with a higher CCI and were more likely to have a history of diabetes, CKD, myocardial infarction, and stroke when compared to statin non-users (P<0.05). However, there were no differences with regard to the presence of dementia, malignancy, Peripheral Vascular Disease (PVD) or congestive heart failure between the two groups (Table 1). Investigation results were similar between the two groups but mean (SD) CRP levels were significantly lower among statin users when compared to statin non-users (74.0(80.8) vs. 121.6 (122.2), P=0.012) mg/L (Table 1).
| Variable | Not on statins | On statins | P value |
|---|---|---|---|
| N (%) | 84 (60) | 56 (40) | |
| Age mean (SD) | 65.3 (19.1) | 74.9 (11.9) | 0.001 |
| Age group n (%) | |||
| <40 | 10 (11.9) | 1 (1.8) | 0.008 |
| 40-59 | 18 (21.4) | 4 (7.1) | |
| 60-79 | 31 (36.9) | 30 (53.6) | |
| >80 | 25 (29.8) | 21 (37.5) | |
| Sex n (%) | |||
| Males | 33 (39.3) | 34 (60.7) | 0.024 |
| Females | 51 (60.7) | 22 (39.3) | |
| Diabetes n (%) | 12 (14.3) | 19 (33.9) | 0.009 |
| CLD n (%) | 5 | 3 (5.4) | 0.572 |
| Malignancy n (%) | 22 (26.2) | 12 (21.4) | 0.303 |
| CKD n (%) | 12 (14.3) | 17 (30.4) | 0.028 |
| CHF n (%) | 12 (14.3) | 13 (23.2) | 0.156 |
| MI n (%) | 10 (11.9) | 20 (35.7) | 0.001 |
| PVD n (%) | 2 (2.4) | 3 (5.4) | 0.379 |
| Stroke n (%) | 4 (4.8) | 10 (17.9) | 0.016 |
| Dementia n (%) | 6 (7.1) | 7 (12.5) | 0.243 |
| COPD n (%) | 20 (23.8) | 22 (39.3) | 0.055 |
| CCI mean(SD) | 4.3 (3.4) | 6.1 (2.9) | 0.002 |
| Hb g/L mean(SD) | 129.1 (19.5) | 123.8 (19.8) | 0.122 |
| WCC x 109/L mean(SD) | 13.0 (6.7) | 12.6 (14.6) | 0.812 |
| CRP mg/L mean(SD) | 121.6 (122.2) | 74 (80.8) | 0.012 |
| Creatinine µmol/L mean(SD) | 102.7 (128.8) | 121.7 (129.9) | 0.396 |
| Troponins ng/L mean(SD) | 25.7 (38.3) | 28.9 (39.4) | 0.631 |
| Elevated troponins n (%) | 16 (34.0) | 11 (33.3) | 0.947 |
| BNP ng/L mean(SD) | 1108.9 (4239.4) | 791.3 (3390.9) | 0.638 |
| Bilirubin µmol/L mean(SD) | 12.6 (13.8) | 11.0 (11.2) | 0.47 |
| AST U/L mean(SD) | 47.2 (76.4) | 58.5 (217.8) | 0.664 |
| ALT U/L mean(SD) | 44.3 (61.4) | 44.8 (140.6) | 0.975 |
Table 1: Baseline characteristics.
Patients who were statin users had a higher severity of pneumonia as reflected by the mean (SD) CURB65 scores when compared to statin non-users (2.0(0.9) vs 1.5(1.2), P=0.0183) (Figure 1), however, individual components of CURB65 scores were similar between the two groups. Overall, 97.1% patients received treatment with antibiotics with the majority of patients receiving a combination of penicillin and azithromycin (48; 34.3%) or cephalosporin and azithromycin (46;32.9%). There were no significant differences in the prescription of individual antibiotics between statin users and non-users (Table 2). There were no differences in the proportion of patients who had MET calls or those who needed ICU admission between the two groups (P>0.05). There was no difference in inhospital mortality between the two groups (2(2.4%) vs 2(3.6%), P>0.05) and 30-day mortality was also similar between the two groups (6(7.1%) vs 5(8.9%), P>0.05).
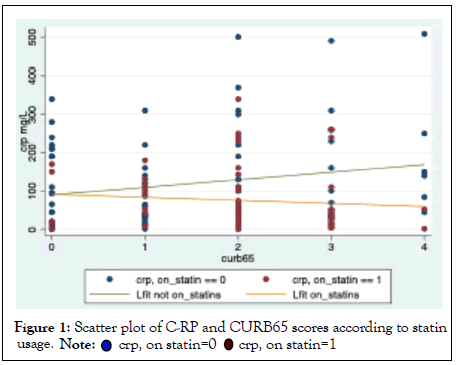
Figure 1: Scatter plot of C-RP and CURB65 scores according to statin usage.
| Variable | Not on statins | On statins | P value |
|---|---|---|---|
| CURB-65 score mean(SD) | 1.5 (1.2) | 2.0 (0.9) | 0.018 |
| RR = 30/min n (%) | 7 (8.3) | 1 (1.8) | 0.09 |
| SBP(mm Hg) mean(SD) | 126.5 (23.2) | 127.0 (21.3) | 0.89 |
| SaO2 mean(SD) | 94.8 (3.3) | 95.6 (2.7) | 0.152 |
| SaO2 = 89% | 7 (8.3) | 3 (5.4) | 0.351 |
| Antibiotics prescribed n (%) | 81 (96.4) | 55 (98.2) | 0.804 |
| Penicillin n (%) | 47 (56) | 32 (57.1) | 0.952 |
| Cephalosporins n (%) | 32 (38.1) | 24 (42.9) | 0.612 |
| Azithromycin n (%) | 51 (60.7) | 41 (73.2) | 0.15 |
| In hospital deaths n (%) | 2 (2.4) | 2 (3.6) | 0.536 |
| MET calls n (%) | 14 (16.7) | 7 (12.5) | 0.299 |
| ICU admission n (%) | 9 (10.7) | 4 (7.1) | 0.462 |
| LOS median (IQR) | 4 (5.0) | 3 (3.0) | 0.717 |
| 30-day mortality n (%) | 6 (7.1) | 5 (8.9) | 0.716 |
| 6-month mortality n (%) | 10 (11.9) | 9 (16.1) | 0.498 |
| 30-day readmissions n (%) | 14 (16.7) | 10 (17.9) | 0.88 |
Table 2: Severity of pneumonia, complications, and outcomes between statin users and non-users.
Logistic regression analyses suggested no differences in either inhospital (aOR 0.840, 95% CI 0.097-7.29, P=0.875) or 30-day mortality (aOR 0.927, 95% CI 0.239-3.599, P=0.914) between the two groups after adjustment for age, sex, Charlson index and CURB65 scores (Table 3). Kaplan-Meier survival curves showed no difference in 30-day mortality between two groups (log rank test chi 20.29, P=0.589) (Figure 2).
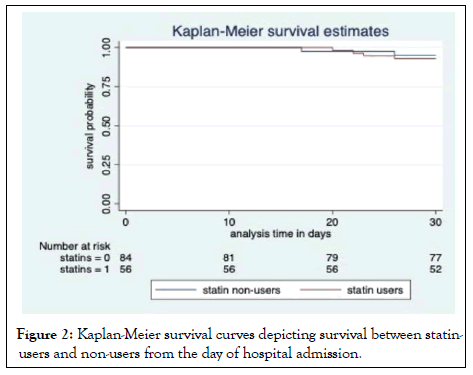
Figure 2: Kaplan-Meier survival curves depicting survival between statinusers and non-users from the day of hospital admission.
There were no significant differences in other clinical outcomes such as LOS, admission to ICU, number of MET calls or 30- day readmissions between the two groups after adjusted analyses (P>0.05) (Table 3).
| Outcome | aOR/Coefficient | 95% CI | P value |
|---|---|---|---|
| In-hospital mortality | 0.84 | 0.097 -7.290 | 0.875 |
| 30-day mortality | 0.927 | 0.239 -3.599 | 0.914 |
| LOS | 0.414 † | -8.2 | 0.842 |
| MET calls | -0.040 † | -0.27 | 0.557 |
| ICU admission | 0.637 | 0.165-2.458 | 0.514 |
| 30-day readmissions | 1.048 | 0.387-2.835 | 0.926 |
Note: †Coefficient
Table 3: Multivariable logistic/linear regression analysis comparing clinical outcomes in statin users with statin non-users as baseline after adjustment for age, sex, Charlson index and CURB65 score.
Propensity score matching created 21 matched subjects between the two groups (Table 4) and no significant differences in in-hospital mortality (average treatment effect coefficient 0.018, 95% CI-0.065- 0.102, P=0.671) or 30-day mortality were observed between the two groups (average treatment effect coefficient-0.017, 95% CI-0.213- 0.1777077, P=0.858). Similarly, we did not observe any significant differences in other clinical outcomes such as admission to ICU, LOS and 30-day readmissions between the two matched groups (P>0.05).
| Variable | Mean not on statins | Mean on statins | Standardised difference (%) | Mean not on statins | Mean on statins | Standardised difference (%) |
|---|---|---|---|---|---|---|
| Total number of patients | 84 | 56 | 21 | 21 | ||
| Age | 65.4 | 74.9 | 60 | 76.6 | 75.1 | 10 |
| Sex | 1.6 | 1.3 | -43.5 | 1.4 | 1.4 | -3.7 |
| Diabetes | 0.1 | 0.3 | 46.8 | 0.4 | 0.3 | -21.7 |
| CAD | 0.1 | 0.4 | 54.5 | 0.3 | 0.4 | 8.5 |
| Stroke | 0.1 | 0.2 | 41.9 | 0.2 | 0.1 | 17.5 |
| Creatinine | 103.5 | 121 | 13.7 | 134 | 122.8 | -9 |
| CURB65 | 1.6 | 1.9 | 35.6 | 1.9 | 1.9 | -5 |
| CCI | 4.3 | 6.1 | 56.2 | 6.1 | 6.1 | 0 |
Table 4: Covariate balance across statin non-users and statin users before and after matching on propensity score.
This study suggests that CAP patients who were on statins at the time of hospital admission were more likely to be older males, with a higher comorbidity burden and cardiovascular risk factors than patients who were not on statins. Statin users had a greater pneumonia severity (as indicated by the higher CURB-65 scores) but displayed lesser degree of inflammation (as indicated by lower CRP levels) than statin non-users. Mortality was similar and there were no differences in other clinical outcomes between the two groups.
Statins and inflammation
The findings of our study suggest that statin users have a lower level of inflammation when compared to statin non-users. The results of our study are similar to a study from UK by Chalmers et al, which included 1007 CAP patients with a mean age of 66 years, 50.3% females and found that median (IQR) CRP levels were significantly lower among statin users when compared to statin non-users (119(46- 215) vs. 176(67-290) mg/L, P ≤ 0.001) [19]. Previous experimental and clinical studies suggest that statins have anti-inflammatory effects. High dose statin therapy has been shown to reduce transendothelial migration of neutrophils in healthy male volunteers [7]. Neutrophil degranulation is involved in the pathogenesis of various vascular inflammatory states. Simvastatin has been previously shown to block both Anti-Neutrophil Cytoplasm Autoantibodies (ANCA) and chemotactic and inflammatory peptide N-Formyl-Methionine-Leucine-Phenylalanine (FMLP) mediated neutrophil degranulation [8]. A double-blind, placebo-controlled study involving 30 healthy subjects demonstrated that simvastatin reduced pulmonary (as per broncho-alveolar fluid lavage assay) and systemic (as per plasma CRP assay) compartment inflammation after being exposed to inhalation of lipopolysaccharide endotoxin, thereby suggesting a possible beneficial role of statins in Acute Lung Injury (ALI) [9]. This suggests that statins have anti-inflammatory effects which might play a protective role in CAP.
Statins and pneumonia severity
In this study we found that statin users had a greater severity of pneumonia when compared to statin non-users. Despite this, adverse events in statin users (measured in terms of the number of MET calls during admission, proportion of patients who needed ICU admission, and mortality) were not significantly higher than statin non-users. The findings of our study are similar to another study which also found that statin users had a greater pneumonia severity as determined by the Pneumonia Severity Index (PSI) when compared to statin non-users and despite this, had a lesser requirement for invasive ventilation or inotropic support during index admission [19]. Similarly, a large population-based study in Taiwan in different ethnic groups demonstrated that statin users who were admitted with CAP had a lower rate of ICU admission when compared to statin non-users [20]. This indicates that statins may have a protective role in CAP. The beneficial effects of statins could be related to the reduced risk of development of complications of CAP such as parapneumonic effusion or empyema as has been observed in a previous study [12,19].
Our study found no differences in relation to mortality or other clinical outcomes such as LOS or readmissions between statin users and non-users. The results of our study are similar to a large multicenter American study which prospectively enrolled 2016 adults with CAP between January 2010 and June 2012 across 5 hospitals and found no differences in mortality (HR 1.03, 95% CI 0.88-1.21) or length of hospital stay (HR 0.99, 95% CI 0.88-1.12) between the two groups [21]. It is important to note that although several previous observational studies have demonstrated a survival benefit in CAP patients who were statin users; this has not been replicated in a randomised control study so far [11-13]. Moreover, it is possible that statin users may have exhibited a constellation of healthy behaviours (the “healthy user effect”) and characteristics which could have been associated with improved outcomes [22]. It is often a challenge to adjust for potential confounders in observational studies because biases may be difficult to characterize, which in turn limits reliability of results.
Strength and Limitations
A major limitation of our study is its small sample size and thus it could have lacked power to detect beneficial effects of statins in CAP. Due to the retrospective design we were unable to gather information about the duration of statin therapy. In addition, we did not measure clinical outcomes in relation to individual statin agents or doses. A major strength of this study is the use of robust statistical measures including the use of propensity scores to balance known confounding factors between the two groups.
This study suggests that, while statin therapy is associated with reduced inflammation among patients with CAP, it is not associated with a reduction in mortality when compared to patients who were not on statins. Further evidence through well designed randomised controlled trials is needed to explore benefits of statins among patients with CAP.
We are grateful to Chris Wong, Jolyn Seah, Sylvia Goedegebuur (Flinders University medical students) and Mona Awadalla (Flinders University research associate), who participated in data collection for this study.
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Australia and New Zealand Clinical Trial Registry (ANZCTR) no 381691
Ethical approval for this study was granted by the Southern Adelaide Human Research Clinical Ethics Committee (SA HREC) no 187.8. All methods were performed in accordance with the relevant guidelines and regulations in the Declaration of Helsinki.
This study received no funding.
Written informed consent was not applicable due to retrospective data collection.
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Sharma Y, Bose S, Horwood C, Hakendorf P, Thompson C (2022) Role of Statins in Clinical Outcomes of Hospitalised Patients with Community Acquired. Intern Med. 12:356.
Received: 14-Jan-2022, Manuscript No. IME-21-15467; Editor assigned: 17-Jan-2022, Pre QC No. IME-21-15467 (PQ); Reviewed: 31-Jan-2022, QC No. IME- 21-15467; Revised: 04-Feb-2022, Manuscript No. IME-21-15467 (R); Published: 11-Feb-2022 , DOI: 10.35248/2165-8048.22.12.356
Copyright: © 2022 Sharma Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.