Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2017) Volume 7, Issue 1
Fast Green FCF is a synthetic food dye. The kinetics study of Fast Green FCF with 1-chlorobenzotiale in HCl medium in presence of RuCl3 catalyst has been spectrophotometrically investigated at ƛmax 625 nm and at the temperature of 301 K. The reaction exhibited a pseudo -first order rate on [FGF] and first order on each of [CBT], [H+] and [Ru(III)]. Negligible effect was observed by the addition of reduced product [BTA] to the reaction mixture. The addition acrylonitrile under the experimental conditions fails to induce the polymerization. Thermodynamic parameters are calculated. The absence of hydride transfer during oxidation reaction was indicated by the observed solvent isotope effect. Effects of ionic strength dielectric constant of the medium on the reaction rate have been also studied. LC-MS spectral data identifies the oxidation products. An appropriate rate law is deduced by the proposed reaction scheme, which is account for the observed kinetic data.
<Keywords: Oxidation; Fast green FCF; 1-Chlorobenzotriazole; Ruthenium chloride; RuCl3 or Ru(III); Kinetics
In organic chemicals, relatively large group of organic synthetic dyes are present in practically all spheres of human life. Dyes make our world beautiful but they also bring pollution and therefore the community has to focus on possible solutions for environmental problems caused by dye industries. Many organic compounds that are toxic and hazardous to human health involved during the manufacturing and the processing of dye. In recent days, due to the increasing presence of refractory molecules in the waste water streams new technologies have been develop to degrade such recalcitrant pollutant molecules into smaller nonhazardous ones. Thus many researchers focused on wastewater treatment. Some methods for wastewater treatment like chemical oxidation, incineration, wet oxidation, air stripping, adsorbents, electrolyte decomposition, ion exchange method, biological methods etc developed by few of the researchers. Due to high cost, these treatment methods are not suitable for large scale and therefore other alternative methods are essential, which are reliable as well as low cost and easy to handle. The heterocyclic dye fast green FCF (Scheme 1) is mainly used in dyeing, prin ting, leather processing, fluorescent pigments and widely used as a colorant in the cosmetics and drug industries. The direct release of wastewater containing fast green FCF causes serious environmental problems due to its dark color and toxicity [1-3]. Fast Green FCF (FGF) is a triarylmethane food dye, which is widely used as food colorant in tinned green peas and other vegetables, jellies, sauces, dishes made of fish, desserts and dry bakery items (In the name of the dye, FCF refers to for Coloring Food). It is a substitute of Light Green SF Yellowish in histology, as its color is less likely to fade. It is used as a protein stain in the electrophoresis. The excess use of this dye induces carcinogenic effects as it produces sarcomas at the site of repeated subcutaneous injection and causes eye, skin and upper respiratory tract irritation. It inhibits the release of neurotransmitter and is an immunotoxic agent [4,5]. The applications and proven toxicity of FGF render it necessary, to develop a sensitive, fast and reliable approach to determine FGF in pharmaceutical, food and biological samples. Owing to concerns over the analytical determination of the dye, number of methods have been developed to analyze the FGF employing variable techniques.
A review of the literature reveals that the detection of this FGF dye by using high performance liquid chromatography [6,7], liquid chromatography-electro spray tandem mass spectroscopy [8], CPE scanometry [9], capillary electrophoresis [10] and spectrofluorimetry [11] were reported. But most of these techniques are suffer from some unfavorable conditions regarding cost, complex sample preparation due to tedious analytical steps and expensive. Moreover, lengthy analysis time is required which make these techniques practically not helpful in routine analysis. In recent times spectrophotometric methods have attracted the attention of researchers because of their sensitivity in the determination of organic molecules, rapidity of analysis, inexpensive and no complex sample pretreatment methods. Conventional evacuation strategies, for example, coagulation/ flocculation, film partition (ultrafiltration, turn around osmosis) or adsorption by enacted carbon, are construct just with respect to a stage exchange of the toxin. As of late, propelled oxidation forms (AOPs) have been created to oxidize the natural compound into CO2, H2O and inorganic particles or into biodegradable mixes [12]. These earth well disposed AOPs are considered as promising wastewater treatment strategies. Here we are revealing the spectrophotmetric strategy for evacuation of poisonous quality of FGF utilizing another oxidant 1-CBT. In spite of the fact that there are no reports on the energy of oxidation of FGF in the writing. A few reviews on the debasement, photodegradation and electrooxidation of FGF have been accounted for by a couple of analysts [13-16]. 1-Chlorobenzotriazole (CBT) is a versatile oxidizing agent and its solution chemistry is reasonably well understood [17]. 1-CBT is an N-halogen compound that contains cyclic three nitrogen chain, has the capacity to undergo certain chemical reactions, which prove its usefulness in organic syntheses. It is soluble in water, chloroform, dichloromethane and ethyle acetate. 1-CBT can be used as a reagent for oxidation and chlorination reactions. Recently, 1 - CBT, has attracted the attention of organic chemists as a novel organic reagent in organic syntheses because of its reactivity towards a number of functional groups. 1-CBT has considerable potential as an oxidant; it oxidizes alcohols to carbonyl compounds, hydrazo- to azo-compounds and 1,1-disubstituted hydrazine, all in high yield under very mild conditions. There are only couple of reports on the kinetics of oxidation of organic compounds by 1-CBT [18-23]. But there are no reports on the kinetics of oxidation of FGF by 1-CBT. With this foundation, we report here outcomes relating to the kinetics and mechanism of oxidation of FGF by 1-CBT. Kinetics of oxidation of FGF by 1-CBT in alkaline medium have been studied in our laboratory and the reaction rate was found to be moderate and measurable without the catalyst. But the kinetics of oxidation of FGF with 1-CBT in acidic medium are very slow and the reaction rate was studied in the presence of ruthenium chloride catalyst.
Experimental
1-CBT Solution: 1-CBT was prepared by the method of Johnson et al. [24]. An aqueous solution of 1-CBT was prepared, standardized periodically by the iodometric method and preserved in an amber colored bottle until further use [25].
Fast green solution: As the substrate, FGF, is prone light, aqueous solutions of FGF (SD. Fine. Ltd) were freshly prepared and used. Fast green (ethyl-[4-[[4-[ethyl-[(3-sulphophenyl) methyl]amino] phenyl]-(4-hydroxy-2 -sulphophenyl)methylidene]-1-cyclohexa-2,5 -dienylidene]-[(3-sulphophenyl)methyl]azanium). Fast green is also known as food green 3, FD and C green No. 3, green 1724, solid green FCF, (C. I. 42053) [26]. Its molecular formula is C37H34N2O10S3Na2, and its molar mass is 808.85 g/mol. stock solution of FGF was prepared in volumetric flask with doubly distilled water.
Deuterium oxide (D2O): Deuterium oxide (99.9 atom % D) was purchased from Sigma-Aldrich in order to study the solvent isotope effect. The other chemicals RuCl3, HCl, Methanol, NaClO4 and NaCl of required concentration were prepared with distilled water and stored as stock solutions.
Kinetic procedure
Active runs were set up under pseudo-first kinetics of [FGF]<<<[CBT] at 301 K [17]. For each run essential measures of of FGF was taken in a stoppered pyrex glass tube whose external surface was covered dark to dispense with photochemical impacts. A required measure of distilled water was added to keep up a steady volume in all runs. The tube was thermally equilibrated at a given temperature. The reaction was started by including a deliberate measure of prequilibrated CBT solution and shaken intermittently for uniform fixation. The progress of the response was observed utilizing UV-VIS. Spectrophotometer LMSP-UV1200 by measuring the absorbance of FGF at its ƛmax of 625 nm at regular intervals of three half-lives. The pseudo-first-order rate constants k, calculated. Regression analysis of the experimental data was completed on a WINDOWS 2007 intel core i5 PC to get the regression coefficient, R2.
Effect of CBT and FGF concentration on the reaction rate
Under pseudo - first request states of [CBT]0>[FGF]0 at consistent temperature, [HCl] and catalyst [RuCl3], the plots of log 2+log absorbance versus time were straight line indicating a first order dependence of the reaction rate of [FGF] (Table 1 and Figure 1). The pseudo first order rate constant k obtained at 301 K are independent of [FGF]0. At steady [H+], temperature, [FGF]0 and [RuCl3], the rate expanded with expanding [CBT]0 (Table 2). Furthermore a plot of log k versus log [CBT]0 was straight line with a positive slope 0.94 indicating first order dependence on [CBT] (Table 2 and Figure 2, R2=0.995). The oxidation of FGF with CBT at constant [FGF], [CBT], [H+] and [RuCl3] and temperature requires 180 minutes, the values of absorbance with regular intervals of time as shown in the Table 1.
| Time in minutes | Absorbance | 2+logabs | k×105s-1 |
|---|---|---|---|
| 0 | 1.0669 | 2.028 | |
| 5 | 1.0328 | 2.014 | |
| 10 | 0.9761 | 1.989 | |
| 15 | 0.9293 | 1.968 | |
| 20 | 0.8976 | 1.953 | |
| 25 | 0.8683 | 1.938 | |
| 30 | 0.8389 | 1.923 | |
| 35 | 0.8135 | 1.910 | 13.40 |
| 40 | 0.7869 | 1.895 | |
| 45 | 0.7654 | 1.883 | |
| 50 | 0.7583 | 1.868 | |
| 55 | 0.7137 | 1.853 | |
| 60 | 0.6968 | 1.843 | |
| 70 | 0.6273 | 1.797 | |
| 80 | 0.5955 | 1.774 | |
| 90 | 0.5657 | 1.752 | |
| 100 | 0.5440 | 1.735 |
Table 1: FGF=2 × 10-5 M; CBT=10 × 10-4 M; HCl=4 × 10-3 M; RuCl3=1.928 × 10-4 M; ƛmax=625 nm and Temp 301 K.
| [FGF]×105M | [CBT]×104M | [HCl]×103M | [RuCl3]×105M | k×105s-1 |
|---|---|---|---|---|
| 1.0 | 10.0 | 4.0 | 19.28 | 13.43 |
| 1.2 | 10.0 | 4.0 | 19.28 | 13.41 |
| 1.4 | 10.0 | 4.0 | 19.28 | 13.25 |
| 1.6 | 10.0 | 4.0 | 19.28 | 13.32 |
| 1.8 | 10.0 | 4.0 | 19.28 | 13.38 |
| 2.0 | 10.0 | 4.0 | 19.28 | 13.40 |
| 2.2 | 10.0 | 4.0 | 19.28 | 13.13 |
| 2.4 | 10.0 | 4.0 | 19.28 | 13.26 |
| 2.0 | 20.0 | 4.0 | 19.28 | 26.73 |
| 2.0 | 30.0 | 4.0 | 19.28 | 35.97 |
| 2.0 | 40.0 | 4.0 | 19.28 | 47.20 |
| 2.0 | 50.0 | 4.0 | 19.28 | 60.53 |
| 2.0 | 60.0 | 4.0 | 19.28 | 76.55 |
| 2.0 | 10.0 | 2.0 | 19.28 | 5.09 |
| 2.0 | 10.0 | 6.0 | 19.28 | 18.03 |
| 2.0 | 10.0 | 8.0 | 19.28 | 22.28 |
| 2.0 | 10.0 | 10.0 | 19.28 | 25.06 |
| 2.0 | 10.0 | 4.0 | 9.64 | 7.44 |
| 2.0 | 10.0 | 4.0 | 14.46 | 9.95 |
| 2.0 | 10.0 | 4.0 | 24.10 | 18.83 |
| 2.0 | 10.0 | 4.0 | 28.90 | 20.46 |
Table 2: Effect of varying concentration of [CBT], [HCl] and [RuCl3] on the reaction rate ƛmax=625 nm and temp 301 K.
Effect of H+ concentration on the reaction rate
The reaction rate increased with increasing H+ concentration (Table 2 and Figure 3, R2=0.964). A plot of log k versus log [H+] was linear with a positive slope of 0.987 showing first order dependence on [H+].
Effect of RuCl3 concentration on the reaction rate
The reaction rate increased with increasing concentration of the catalyst RuCl3 (Table 2 and Figure 4, R2=0.974). A plot of log k versus log [Ru3+] was a straight line with a positive slope of 0.98 showing first order dependence on [Ru3+].
Reactive species of RuCl3
RuCl3 catalysis in redox reactions involves different degrees of complexicity, due to formation of different intermediate complexes, free radicals and its variable oxidation states. Cady and Connick [27-33], and Connick and Fine [34] have investigated aqueous RuCl3 complex species using the ion- exchange resins and UV-spectral studies. They found that the octahedral complex species [RuCl5(H2O)]2- [RuCl3(H2O)3], [RuCl2(H2O)4]+ and [RuCl(H2O)5]2+ may not exist in aqueous solution of RuCl3. Other studies [35-37] have shown the existence of following equations for RuCl3 in acidic solutions:
RuCl3xH2O+3HCl → [RuCl6]3-+xH2O+3H+ (1)
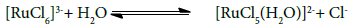 (2)
(2)
In the present study, the absence of chloride ion effect on the rate indicates that the equilibrium (2) does not play any role in the reaction. Hence, the complex ion, [RuCl5(H2O)]2-, is assumed to be the reactive catalyst species. The observed rates of oxidation FGF dye in the presence of RuCl3 catalyst revealed that the reactions are about two-fold faster than the uncatalyzed reaction. This might be credited to the development of the intermediate complex X1 between RuCl3 and the X, which increases the rate with RuCl3 catalyst. Consequently, it can be said that RuCl3 is an efficient catalyst for the present redox system.
Effect of reduction product [BTA] on the reaction rate
The effect of added reduction product, BTA on the rate keeping other experimental conditions same was studied in the range -1 × 10-4 M to 4 × 10-4 M. Negligible effect was observed by the addition of [BTA]. The results are tabulated in Table 3.
| [BTA]×105(M) | K×105(s-1) |
|---|---|
| 0.0 | 13.40 |
| 10.0 | 13.58 |
| 20.0 | 13.0 |
| 30.0 | 13.14 |
| 40.0 | 13.88 |
Table 3: Effect of varying Benzotriazole (BTA) Concentration on the Reaction Rate. [FGF]=2 × 10-5 M; [CBT=10 × 10-4; [HCl]=4 × 10-4; [RuCl3]=19.28 × 10-5 ƛmax= 625 nm and temp 301 K.
Effect of dielectric constant (D) on the reaction rate
In the present study the methanol effect on the reaction rate is in conformity with the Amis concept of dipole-dipole interaction or dipole-ion interactions [27]. In order to find out the nature of reactive species, the dielectric constant (D) of the medium was varied by adding different amounts of methanol (0-30% v/v) to the reaction mixture. The rate decreased with increasing methanol content (Table 4 and Figure 5, R2=0.995). Several approaches [38-42] have been made to explain quantitatively the effect of dielectric constant of the medium on the rates of reactions in solutions. Amis et al. [39] predicted a linears relation between log k0 versus 1/D. The slope of such a plot should be negative for a reaction between a negative ion and a dipole or between two dipoles, while a positive slope obtained for positive ion–dipole reactions. In the present investigations, plots of log k0 versus 1/D were linear with negative slopes, thus supporting the participation of the two dipoles in the rate-determining step (Scheme 4).
| MeOH(%) | Dielectric Constant(D) | K×105(s-1) | 1/D | 5+logk |
|---|---|---|---|---|
| 0 | 76.73 | 13.40 | 0.0130 | 1.127 |
| 10 | 72.37 | 12.64 | 0.0138 | 1.102 |
| 20 | 67.48 | 11.85 | 0.0148 | 1.074 |
| 30 | 62.71 | 11.19 | 0.0159 | 1.049 |
Table 4: Effect of varying Methanol Concentration on the Reaction Rate. [FGF]=2 × 10-5 M; [CBT=10 × 10-4; [HCl]=4 × 10-4; [RuCl3]=19.28 × 10-5 ƛmax=625 nm and temp 301 K.
Effect of ionic strength and halide ion on the reaction rate.
Ionic strength of the reaction medium was altered by adding NaClO4 (0.001 to 0.1 mol dm-3). There was no change in the rate of reaction. The reaction mechanism has been proposed based on the observed zero effect of ionic strength on the rate of the reaction. The primary salt effect on the reaction rate has been described by Bronsted and Bjerrum theory [38]. In the present investigation, neutral molecules are involved in the rate determining step. Hence, variation of the ionic strength of the medium does not alter the rate in confirming of the above theory [38]. Addition of halide ion (0.4 × 10-3 to 4 × 10-3 mol dm-3) in the form of NaCl to the reaction mixture also had negligible effect on the rate.
Effect of temperature on the react ion rate and activation parameters
Kinetic runs were performed at various temperatures from 301K to 313K while keeping other experimental conditions same. An Arrehenius plot of log k versus 1/T (Table 5 and Figure 6, R2 =0.999) was used to calculate the activation parameters, namely energy of activation (Ea), entropy of activation (ΔS#), enthalpy of activation (ΔH#) and free energy of activation (ΔG#). These results are shown in Table 4 and Figure 6. The values of ΔH# indicate that the reaction is enthalpy controlled. The more positive values of ΔG# points out a highly solvated transition state. The large negative values of ΔS# signifies that the transition state is more rigid than the initial state with less degrees of freedom. The values of frequency factor (log A) specify the frequency of collisions and orientation of the reaction molecules.
| Temp in K Parameters | K×105S-1 | Activation |
|---|---|---|
| 301 | 13.40 | Ea:67.93KJ/mol |
| 305 | 19.58 | DH#:65.43KJ/mol |
| 309J/K/mol | 26.38 | DS#:-101.62 |
| 313 | 38.01 | DG#:34.83KJ/mol |
| 317 | 52.23 | logA:8.92 |
Table 5: Effect of temperature on the reaction rate. [FGF]=2 × 10-5 M; [CBT=10 × 10-4 M; [HCl] =4 × 10-4 M; [RuCl3]=19.28 × 10-5 M; ƛmax=625 nm.
Reaction stoichiometry
Differing proportions of the oxidant (CBT) to FGF in acidic medium were equilibrated at 301 K for 48 hrs. Aliquots of the reaction mixture were idometrically titrated with a standard thiosulphate solution, utilizing starch marker, to decide the centralizations of unaltered CBT. The mole proportion (number of moles of CBT expended per mole of FGF) was ascertained. The consequences of FGF response with CBT demonstrated a distinct stoichiometry of 1:1 (Scheme 2).
Product analysis
The reaction mixture in the stoichiometric ratio was allowed to progress for 48 h in presence of HCl and RuCl3 at 301 K under stirred conditions. After completion of the reaction (monitored by TLC), the reaction products were extracted with ethyl acetate. The oxidation products were confirmed by LC–MS analysis. The oxidation product of FGF, are sodium 3-hydroxybenzenesulfonate and sodium 3-(2-(3-(4-(ethyl(3-sulfobenzyl)amino)benzoyl)phenyl)butyl) benzenesulfonate. The mass spectra showed a molecular ion peak at 630 and 197 amu, clearly confirming the benzene sulfonate derivatives as the oxidized products. The reduction product of CBT, benzotriazole (BTA) was isolated and identified by TLC using butanol-acetic acid-water (4:1:1) as solvent and iodine as the detecting agent (Rf=0.92). The obtained Rf value was consistent with literature value [28]. It was also noticed that there was no further reaction of these oxidation products under the present set of experimental conditions (Figure 7 and Scheme 3).
Reaction mechanism
The probable mechanism is proposed, based on the experimental results obtained (Scheme 4).
Oxidation of Fast green FCF was observed at λmax = 625 nm. A graph plotted between log Abs v/s time was a straight line, which shows that oxidation of fast green FGF with 1-CBT follows the pseudo-first order kinetics. The data of typical run have been presented in Table 1 and also graphically represented in Figure 1 respectively.
Reactive species of CBT
1-CBT being N-haloamine gives several oxidizing species in aqueous solution. The concentration of each species depends on the concentration of 1-CBT, the nature (polar or nonpolar) and pH of the medium. Benzotriazole (BTA), the parent compound of CBT, has a pKb value of 5.8 and hence it might largely exist in protonated form in an aqueous solution [29].

Because of the weakening of the N–Cl bond, it might solvalize further to give H2OCl+,
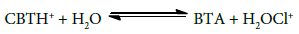
H2OCl+ may generate Cl+ on cleavage [31]. But, it rarely participates in the oxidation reaction under the experimental conditions [32]. Therefore, the possible oxidizing species in acidified CBT solution are: CBT, CBTH+ and H2OCl+. If H2OCl+ is the active oxidant species, a retardation of rate by added BTA is expected [30]. However, no such observation was found, hence CBT or CBTH+ is assumed to the reactive species for FGF kinetics. The following reaction mechanism appears plausible to account for the rate law and showed that CBT is assumed to be reactive species (Scheme 5).
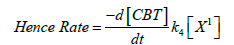 (1)
(1)
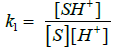 (2)
(2)
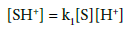 (3)
(3)
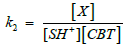 (4)
(4)
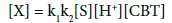 (5)
(5)
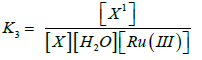 (6)
(6)
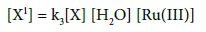 (7)
(7)
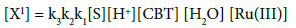 (8)
(8)
Substitute the value of [X1] in rate law
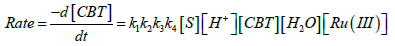 (9)
(9)
The rate law (9) is in close concurrence with the trial comes about that pseudo first order for [FGF] and first order on each for [CBT], [H+] and [Ru(III)]. Solvent isotope studies in D2O medium show an increase in the reaction rate. It is well known that D3O+ is a stronger acid [41-43]. than H3O+ and hence this observation supports the proposed mechanism. The insignificant impact of BTA on the rate of reaction shows that it was not included in pre-balance. Expansion of acrylonitrile to the reaction mixture had no impact on the reaction rate, showing the nonappearance of free radical species amid the oxidation reaction. The change in the ionic strength of the medium during the oxidation reaction by using NaClO4 did not alter the rate, indicating that non-ionic species involved in the rate determining step. A slight negative dielectric constant effect on the rate supports the fact that the dipole-dipole interaction in the rate determining step. The proposed mechanism is supported by the moderate values of energy of activation and other thermodynamic parameters. The fairly high positive values of free energy of activation and enthalpy of activation indicate that the transition state is highly solvated, while the high negative entropy of activation suggests the formation of compact activated complex with fewer degrees of freedom. Rate law 9 is in accordance with the experimental findings. The proposed schemes and the derived rate law are also substantiated by the experimental observations discussed.
Fast Green FCF is a synthetic food dye which is part of an important class of organic compounds originally synthesized from coal tar and from petroleum. These food dyes are commonly used as additives in food, medicines and soft drinks to get suitable and natural looking colors, because of their greater brightness, stability, low cost and wide ranges of shades. Consequently, they have become common industrial and environmental pollutants during their synthesis and applications. Some of the artificial coloring ingredients are toxic to our health and environmentally hazardous. There were no reports on the kinetics of oxidation of FGF dye with any oxidants in the literature. Here we are reporting the spectrophotmetric method of kinetics of oxidation and removal of toxicity of FGF using a new oxidant 1-CBT.
The authors greatly acknowledge the help of IOE, University of Mysore, Mysuru for the spectral study. Also, the authors thank Prof. S. Ananda, Chairman, Department of Studies in Chemistry, University of Mysore, Mysuru for his valuable suggestions regarding the reaction mechanism scheme.