Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Research Article - (2022)Volume 12, Issue 2
The main aim of this work was to develop an effective method for pretreatment and enzymatic saccharification of cellulose and hemicellulose components into fermentable sugars, as well as fermentation of the hydrolysate to bioethanol by new yeast isolates. YAJ-2M (3.2%), YCJ-1 (3.4%) and YAJ-2M 11 (5.6%) produced maximum ethanol. UV- induction responsible for genetic improvement of yeast isolates for ethanol production. Pretreatment of paper with alkali- acid reduced the lignin content from 25% to 14.3%. Maximum liberation of reducing sugars (168.7 μg/ ml) was attained at 50°C for 96 hours at 150 rpm using enzyme dose of 20 U/g in alkali-acid (sodium hydroxide- orthophosphoric acid) pretreated paper cup. Fermentation of hydrolysate paper by yeast isolates (YAJ-1 M2 11 mutant) at 30°C, pH 4.7, 150 rpm and resulted in 1% ethanol after 96 h. Present investigation was found mutant YAJ-1 M2 11 produced highest ethanol concentration in hydrolysed paper than other tested mutant.
Yeast isolates; Paper cup; Ethanol; Saccharification; Fermentation
Due to energy crisis world-wide, depletion of non-renewable fuel sources and growing environmental problems, there is intense need in optimizing fermentation processes for the large-scale production of alternative renewable sources of bioenergy [1]. The largest potential feedstock for ethanol is lignocellulosic biomass, which includes materials such as agricultural residues, herbaceous crops, short rotation woody crops, forestry residues, waste paper and other plant waste. Lignocellulosic biomass varies among plant species but generally consists of 25% lignin and 75% carbohydrate polymers. Lignocellulosics are the largest known renewable carbon source that can be converted into biofuels such as ethanol with reduced emission of greenhouse gases [2]. Recent developments in both cost and increasing demand for crude oil have motivated a world-wide research for low cost, easily available and relevant feedstock for large scale production of alternate bioenergy sources [3]. Various pretreatment strategies are strictly used to enhance the liberation of fermentable sugars from agricultural residues, as they increase the accessible surface area for enzymatic digestability by disrupting or eliminating lignocellulosic constituents [4]. The recalcitrant nature of lignocellulosic biomass have been minimizing by different pretreatment strategies [5]. The crystalline structure of lignocellulosic converts into amorphous form without any consequential energy requirement [6]. Enzymatic saccharification of lignocellulosic materials by cellulases for biofuel production is the most popular application [7]. The pretreatment usually enhances the enzymatic accessibility of biomass by reducing particle size with the aim of producing substrates that can be more rapidly and efficiently hydrolysed to yield mixture of fermentable sugars [8].
Alkaline pretreatment is usually preferred in comparison to other chemicals because it dissociates the ester bonds between cellulose, hemicellulose and lignin, resulting in effective delignification of biomass [9,10]. Bioethanol is an inexhaustible renewable energy source that act as a viable alternative of fossil fuels. In India, ethanol is produced by mainly the fermentation of molasses using Saccharomyces cerevisiae. Species that can efficiently utilize both pentoses and hexoses are less efficiently at converting sugars to ethanol. Saccharomyces cerevisiae can be improved by metabolic engineering for xylose utilization. Besides this information the emphasis will be laid on improving ethanol production using genetically engineered strains. While the first generation ethanol is restricted on starchy food materials, production of second generation ethanol from lignocellulosic biomass from major food crops is sufficient to meet global demand for liquid fuels. Present study involved the optimization of pretreatment of paper cup by chemical methods followed by saccharification of pretreated biomass for ethanol production by yeast isolates.
Yeast isolation and culture conditions
Yeasts were isolated from fruit juices. 100 μl of each sample was added in test-tubes containing 5 ml sterile YEPD broth containing (w/v %) yeast extract 1.0, peptone 2.0, dextrose 2.0 (pH 4.8). The tubes were incubated at 28°C for 48 hours. After growth, a loopful inoculum from each tube was streaked on Yeast Extract Peptone Dextrose (YEPD) agar media was examined and YEPX agar medium containing (w/v %) yeast extract 1.0, peptone 2.0, xylose 2.0, agar 2.0 (pH 4.8). The plates were incubated at 28°C until yeast colonies were developed.
Maintenance of culture
The stock cultures of yeast was maintained by subculturing on slants using YEPD and YEPX, incubating for 48 hours at 30°C, and thereafter, storing in a refrigerator at -4°C for growth and production, 24 hours old slant culture was used for the preparation of inoculum.
Screening of yeast isolates for xylose utilization
The ability to ferment D-xylose was tested in tubes containing YEPX broth and incubated at 28°C on shaker for 24 hours then tubes were kept at stationary condition for 48 hours. The yeast cultures were checked for their ability to utilize the pentose sugar (xylose) by plating the isolates on YEPA containing 2% xylose.
Determination of ethanol producing ability of different yeast isolates
Ethanol was produced by different yeast isolates in dextrose medium. For this, YEPD broth was prepared containing (w/v %) yeast extract 1.0, peptone 2.0, dextrose 8.0 (pH 4.8) and inoculated with yeast cultures. The tubes were kept on the rotary shaker at 30°C at 100 rpm for 24 hours for carrying out fermentation process. After 48h, the culture broth was analysed for ethanol production.
Estimation of ethanol and sugars
Ethanol was estimated colorimetrically by the method of Caputi et al. The sugar released was estimated using DNS method. 0.5 ml of sample (20 μl sample+480 μl distilled water), 0.5 ml of DNS regent was added.
Genetic improvement of selected yeast isolates for improved ethanol production
The promising isolates producing higher levels of ethanol in fermentation broth were selected and further treated. Their genetic improvement was carried out by using the physical mutagen (UV irradiations). Homogenous cell suspensions of the selected isolates were prepared and 50 μl of each of these suspensions were inoculated on YEPD agar medium using spread plate technique. One plate was irradiated with 30 Watt UV germicidal lamp with maximum output at 2537 Ǻ at a distance of 40 cm for 30 min. Another plate was kept as control. Both the plates were incubated at 28°C for 48 hours.
Pretreatment of paper for extraction lignin
The amount of cellulose, hemicellulose, and lignin in untreated and pretreated biomass was determined. The effect of alkaline pretreatment depends on the lignin content of the materials. The concentration of NaOH was used as per the amount.
Saccharification of paper
Pretreatment of paper (solid loading of 10% w/v) was carried out with sodium hydroxide (1.25% w/v) at 120°C for 15 minutes. Pretreated solid biomass was washed and suspended in orthophosphoric acid (70% w/v) for 3 hours. After 3 hours, the acid was drained off. Solution was filtered and washed with acetone followed by repeated washing with distilled water until the pH increased to 4.8. Pretreated paper was suspented in citrate buffer (0.05 M) pH 4.8. In preliminary experiment, saccharification of pretreated biomass was optimized by cellulase enzyme dose of 20 U/g at 50°C for 96 hours at 150 rpm (shaking condition) and were estimated for reducing sugar (using DNS Method for sugar estimation) after every 24 hours for 4 days.
Screening of UV induced mutants for ethanol production using dextrose, xylose, and pre-treated lignocellulosic paper
The most promising mutants of the isolated strains were selected for further studies. YEP broth (8% dextrose) dispensed 5 ml in test tubes and sterilized. These tubes were inoculated with the desired strain and incubated at 28°C for 48 hours with shaking for 24 hours and under static conditions for next 24 hours and ethanol was estimated after 48 hours. Yeast biomass settled at the bottom of the tubes and the medium at the top was drained off under aseptic conditions in the laminar air flow. Fresh YEP medium containing 6% xylose was added to these tubes containing yeast cells. Tubes were incubated in the similar fashion as in the case of dextrose and ethanol was estimated after 24 hours. Then, the pretreated paper was inoculated with pure yeast culture of the selected isolates and was incubated at 28°C under static conditions.
Isolation of yeasts
In the present study yeast cultures were isolated from different fruit juice samples showed in Figure 1. A total of 5 different isolates were isolated from all collected fruit juice (Anar, Carrot, Orange, Grapes, Pine apple) and named as Yeast Anar Juice strain (YAJ-1), Yeast Carrot Juice strain (YCJ-1), Yeast Orange Juice strain (YOJ- 1), Yeast Grapes Juice strain (YGJ-1) and Yeast Pineapple Juice strain (YPJ-1). The isolates were grown on dextrose medium. The utilization of dextrose by yeast cells showed that dextrose is an appropriate substrate for fermentation as shown in Table 1.
Figure 1: Wet mount of yeast cells.
| Source | Yeast isolated | Growth on YEPD plate |
|---|---|---|
| Anar | YAJ-1 | + |
| Carrot | YCJ-1 | + |
| Orange | YOJ-1 | + |
| Grapes | YGJ-1 | + |
| Pine apple | YPJ-1 | + |
Note: +=Growth on the YEPD plate
Table 1: Natural source used for yeast culture isolation
Microscopic observation.
The cell morphology of the pure cultures observed under the microscope (100X magnification) by using Negative Staining Technique showed large, elliptical budding and non-budding yeast cells. The budding stage of the isolates was observed after incubation for 48 hours at 30°C and confirmed to be yeast as shown in Figure 2.
Figure 2: Yeast cells examined under 100X magnification.
Screening of yeast isolates for sugar utilization
The pure isolated colonies were grown on YEPD and YEPX media (28°C) were screened for utilization of dextrose and xylose in Table 2. The growth of the isolated colonies was observed after 48 hours of incubation. Isolates-YAJ-1, YCJ-1 and YOJ-1 were found to utilize xylose for their growth at a faster rate as compared to the Isolate-YGJ-1.
| Culture | Dextrose utilization | Xylose utilization |
|---|---|---|
| YAJ-1 | ++ | ++ |
| YCJ-1 | ++ | ++ |
| YOJ-1 | ++ | ++ |
| YGJ-1 | ++ | + |
Note: +=Growth on the basis of different sugar utilization
Table 2: Primary screening of yeast isolates for dextrose and xylose utilization.
Screening of the isolated yeast strains for ethanol production
Cultures were incubated in BOD incubator at 30°C under stationary conditions after 72 hours ethanol production was analysed in culture broth. The optical density was taken at 600 nm by calorimetric method by using the spectrometer. From the ethanol production study the ethanol production levels of all the strains were found to be in the range of 0.5% to 3.6% Table 3. YCJ- 1 isolate produced maximum amount of ethanol (3.6%), more biomass observed at the bottom of the tubes in comparison to other isolates YAJ-1(1.0%) and YOJ-1(0.5%).
| Yeast isolate | *Growth | % Ethanol (v/v) |
|---|---|---|
| YAJ-1 | +++ | 1 |
| YCJ-1 | ++++ | 3.6 |
| YOJ-1 | ++ | 0.5 |
Note: *=Based on the culture pellet at the bottom of the tubes
Table 3: Primary screening of yeast isolates for dextrose and xylose utilization.
Genetic improvement for yeast isolates for the improved ethanol production using UV irradiation
UV radiation generates cyclobutane type dimers, usually thymine dimers, between adjacent pyrimidines. The chance of forming multiple mutations is reduced, which therefore increase the frequency of isolating strains differing at a single locus but which are otherwise isogenic. After UV induction, different isolates were observed in Table 4 in different morphology. YAJ-1 consists of 12 colonies on plate, these colonies were raised and white in colour. YCJ-1 and YOJ-1 isolates showed 7 and 8 colonies respectively on plate. These colonies were soft and creamy white in colour. YAJ-1 showed more colonies than other isolates which means % survival for this isolate was more than the other ones. S. cerevisiae was not utilized pentose sugars for ethanol production. Yeasts can be improved by genetic engineering to utilize both hexoses and pentoses sugars.
| Source (fruit juice) | Yeast isolate (wild type) | Uv-induced (no. of colonies) | Morphology |
|---|---|---|---|
| Anar | YAJ | 12 | Raised and White in colour |
| Orange | YOJ | 8 | Soft and Creamy white in colour |
| Carrot | YCJ | 7 | Soft and Creamy white in colour |
Table 4: After UV- Induction different isolates were observed different morphology.
Screening of UV Induced mutants of yeast isolates for ethanol production in dextrose
UV-induction responsible for genetic improvement of yeast isolates for ethanol production. The ability to ferment dextrose was tested in tubes containing 8% (w/v) solution of the sugar. We were mutated isolates of YAJ, YCJ and YOJ used for production of high amount of ethanol in dextrose and xylose medium. Mutants of the isolates YCJ and YOJ did not produce high amount of ethanol as compared to its parent type. Ethanol production from xylose is a rare phenomenon among yeasts. 200 species tested the laboratory, only 6 (such as Pichia stipites, Candida shehatae) strains accumulated ethanol to more than 1 g/L.
UV- induced mutant of yeast isolate YAJ-1 (Yeast Anar Strain)
After incubation period, 12 mutants of YAJ-1 were observed on YEPD plate. These mutants were raised, round and white in morphology. These mutants were kept in shaker for 24 hours and again for next 48 hours in static condition. Ethanol produced after incubation was found to be 3.2%, 2.5% and 1.7% by M2, M3 and M1 mutants respectively. The mutants M9, M8, M12 and M5 showed no ethanol production even though the biomass production was high in their case, the mutants were down regulated. YAJ-1 M2 was selected for re-expose to UV light because they produced highest amount of ethanol in 1st generation. After 2nd generation mutation, 16 colonies were observed in Table 5 which produced comparatively high amount of ethanol than the wild types. Some mutants produced high amount of ethanol like M9, M10, M11, M15, and M16 where M11 mutants produced highest amount of ethanol (5.6%) in the medium.
| 1st Generation mutants | %Ethanol (V/V) | 2nd Generation mutants | %Ethanol (V/V) |
|---|---|---|---|
| YAJ-M1 | 1.7 | YAJ-M2 Parent type | 3.2 |
| M2 | 3.2 | M2 M1 | 4.2 |
| M3 | 2.5 | M2 | 4.3 |
| M4 | 0.8 | M3 | 4.4 |
| M5 | NEP | M4 | 4 |
| M6 | NEP | M5 | 3.9 |
| M7 | 0.6 | M6 | 3 |
| M8 | NEP | M7 | 3.5 |
| M9 | 0.09 | M8 | 3.7 |
| M10 | NEP | M9 | 5 |
| M11 | NEP | M10 | 4.7 |
| M12 | NEP | M11 | 5.6 |
| M12 | 4 | ||
| M13 | 3.5 | ||
| M14 | 3.5 | ||
| M15 | 5 | ||
| M16 | 5 | ||
Note: NEP=No ethanol production by strain
Table 5: Screening of UV- Induced mutants of yeast isolate of anar YAJ-1 (1st generation) and YAJ-1 M2 (2nd generation) for ethanol production.
UV- Induced mutant of yeast isolate YCJ-1 (Yeast Carrot Strain)
The 7 mutants (YCJ- 1) observed after UV irradiation did not produce high amount of ethanol than wild type. After re-exposure of isolate- YCJ- M1 to UV- light, 7 mutant colonies were shown in Table 6 on the petri plate. Mutants of YCJ- M1 did not produce higher amount of ethanol than its wild type. All the mutants were downregulated because the mutants were not produced highest amount of ethanol than wild type.
| 1st Generation mutants | % Ethanol (V/V) | 2nd Generation mutants | % Ethanol (V/V) |
|---|---|---|---|
| YCJ-M1 | 3.4 | YCJ-M1 Parent type | 3.4 |
| M2 | 3 | M2 M1 | 3 |
| M3 | 3.4 | M2 | 1.7 |
| M4 | 2.9 | M3 | 1.6 |
| M5 | 3.5 | M4 | 1.7 |
| M6 | 2.6 | M5 | 2 |
| M7 | 2.8 | M6 | 2.9 |
| M7 | 2.8 | ||
Table 6: Screening of UV- Induced mutants of yeast isolate of carrot YCJ-1 (1st generation) and YAJ-1 M1 (2nd generation) for ethanol production.
Screening of yeast isolate in xylose medium for ethanol production
The ability to ferment xylose was tested in tubes containing 6% (w/v) solution of the sugar. The tubes were incubated at 28°C in an orbital shaker at 80 rpm for 24 hours at static conditions. 0.6, 0.7 and 0.3% of ethanol produced in the xylose medium by mutants of isolates YAJ-M2 16, YAJ-M2 12 and YAJ-M2 9 observed in Table 7. Ethanol production in xylose medium was observed in fewer amounts in comparison to dextrose medium observed in fewer amounts in comparison to dextrose medium YAJ- M2 showed an increased ethanol production as compared to their wild type culture, upon UV induction.
| Culture | Growth in xylose | % Ethanol |
|---|---|---|
| YAJ-M2 | +++ | 0.2 |
| YAJ-M2 9 | +++ | 0.3 |
| YAJ-M2 10 | ++ | 0.5 |
| YAJ-M2 11 | +++ | 1 |
| YAJ-M2 12 | +++ | 0.7 |
| YAJ-M2 16 | +++ | 0.6 |
| YCJ-1 | ++++ | NEP |
Note: NEP=No ethanol production by strain
Table 7: Primary screening of yeast isolates for dextrose and xylose utilization.
Effects of alkali-acid pretreatment on chemical composition of paper
Lignocellulosic biomass varies among plant species but generally consists of 25% lignin and 75% carbohydrate polymers. Alkali- Acid pretreatment reduced the lignin content from 25% to 14.3% showed in Table 8. Alkaline pretreatment resulted in delignification in various agro-residues i.e., rice straw, Eucalyptus and others. In the present study, cellulose contents as high as 57% and as low as 38%, with lignin in the range of 17-37% by weight as shown in Figure 3.
| Amount of paper (g) | Holocellulose (g) | Lignin (g) | %Lignin |
|---|---|---|---|
| 80 | 68.55 | 11.45 | 14.313 |
| 10 | 8.57 | 1.43 | 14.3 |
Table 8: Yield of holocellulose and lignin in paper.

Figure 3: Hydrolysis of paper cup.
Saccharification of paper for estimation of reducing sugars
The portion of lignin after extraction, consists of holocellulosic. The holocellulosic was further treated with orthophosphoric acid (70% w/v) for saccharification of pretreated (alkali-acid) paper in citrate buffer (0.05 M) pH 4.8 by cellulase enzyme dose (20 U/g) at 50°C for 96 hours at 150 rpm (shaking condition) was further carried out for different time intervals that resulted in high amount of reducing sugars (168.7 μg/ml ) after 96 hours in Table 9. Residual sugars released from the hydrolysed paper this sugar used by yeast isolate to conversion of bioethanol. Saccharification of alkaline pretreated rice straw by crude fungal cellulases from trichoderma harzianum SNRS3 that resulted in 29.87 mg/ml reducing sugars. Enzymatic hydrolysis of ammonia pretreated oil palm fronds resulted in enhanced glucose yield with an increase in enzyme loading from 15 to 150 FPU/g at 50°C after 96 hours.
| Time (hour) | Reducing sugar (μg/ml) |
|---|---|
| 0 | 45.5 |
| 24 | 54.5 |
| 48 | 90.29 |
| 72 | 110 |
| 96 | 168.7 |
Table 9: Residual sugar released from the hydrolysed paper.
Fermentation profile
In fermentation profile, sugar was utilized by microorganism to production of ethanol. Sugar was reduced due to increase time period and ethanol yield increase. Fermentation of hydrolysate of alkali-acid (NaOH-Orthophosphoric) pretreated paper by yeast isolates (YAJ-1 M2 11 mutant) at 30°C, pH 4.7, 150 rpm and resulted in 1% ethanol after 96 h in Figure 4. This mutant was produced highest amount of ethanol in hydrolysed paper than other mutant. YAJ-1 M2 9 mutant produced .7% ethanol in Figure 5 and mutant YAJ-1 M2 2 mutant produced 0.8% ethanol in Figure 6 hydrolysed paper. SSF (simultaneous saccharification and fermentation) of ammonia pretreated rice straw by S. cerevisiae (ATCC 96581) at 37°C, 150 rpm and reported 84% ethanol yield in 50 g/L of substrate after 72 hours. Ammonia pretreated enzymatic hydrolysate (10%) was fermented by S. cerevisiae and 20% hydrolysate resulted in 24.37 g/L ethanol after 72 hours. 18.6 g/L ethanol in ammonia pretreated empty fruit bunches in SSF.
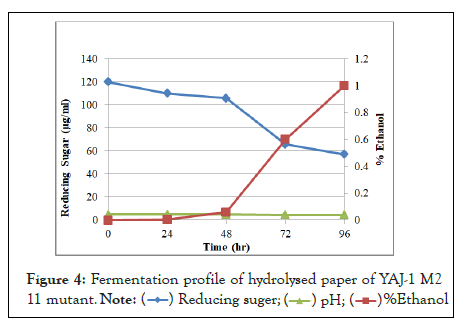
Figure 4: Fermentation profile of hydrolysed paper of YAJ-1 M2 11 mutant. 
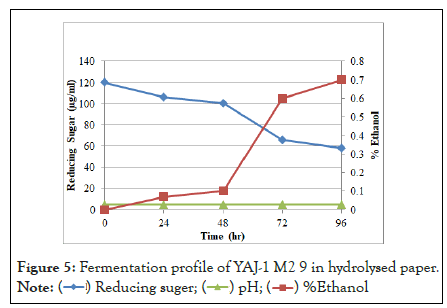
Figure 5: Fermentation profile of hydrolysed paper of YAJ-1 M2 11 mutant. 
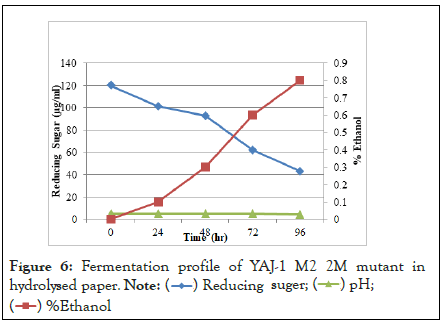
Figure 6: Fermentation profile of YAJ-1 M2 2M mutant in hydrolysed paper. 
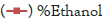
The present studies on “Saccharification of used paper cup for ethanol production by yeast isolate.” The yeast isolates from fruit juice were grown for 48 h in dextrose and xylose medium for utilized sugars to conversion into ethanol. All the isolates were utilized dextrose for metabolism growth that means dextrose used as substrate for fermentations. The fermentation of sugars to ethanol by yeast isolates was maximum at 48-72 hours incubation at 28-30°C under stationary conditions. YAJ-2M (3.2%), YCJ-1 (3.4%) and YAJ-2M 11 (5.6%) produced maximum ethanol. Studies carried out using acid-alkali treatment for hydrolysed paper of liberating reducing sugars (168.7 μg/ml) at 96 h from pretreated biomass for bioethanol. Lignified cellulose and hemicelluloses to efficient processing and liberation of the fermentable sugars for ethanol. Alkali-Acid pretreatment increased the holocellulose content from 83% to 85.68% while reduced the lignin content from 17% to 14.3%. Hydrolysate substrate of pretreated paper was fermented to ethanol (1% v/v) by yeast isolate (YAJ-2M 11) at 30°C, pH 4.7, 150 rpm after 96 h. Result indicated that hydrolysate substrate produced ethanol by inoculated yeast culture into substrate. High amount of ethanol produced into substrate by YAJ-2M 11.
Citation: Kumari N, Komal, Gulia SS, Ram K (2022) Saccharification of Used Paper Cup for Ethanol Production by Yeast Isolate. Fungal Genom Biol. 12:185.
Received: 11-Mar-2022, Manuscript No. FGB-22-17172; Editor assigned: 15-Mar-2022, Pre QC No. FGB-22- 17172 (PQ); Reviewed: 29-Mar-2022, QC No. FGB-22-17172; Revised: 04-Apr-2022, Manuscript No. FGB-22-17172 (R); Published: 11-Apr-2022 , DOI: 10.35841/2165-8056.22.12.185
Copyright: © 2022 Kumari N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.