Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2022)Volume 10, Issue 6
Objective: To analyse historical indications, tolerance, secondary effects and effectiveness of a treatment regimen with short interrupted cycles of large doses of PGE1 as well as possible factors that may influence the results in real world clinical practice in a tertiary hospital.
Material and Methods: This is an observational, descriptive, retrospective study of all patients treated from 2013 to 2020 with high-doses of PGE1 in our hospital.
Results: Forty five patients from 27 to 91 years old were treated for ischemic diseases, grouped into 6 types. Treatment caused side effects in 14 patients (31%) and has to be discontinued in 6 of them for intolerance (13% of total). Intolerance was associated with heart disease and advanced age. No short or long term complications were observed in these patients after discontinuation of treatment. Effectiveness was considered in 53.3% in mean, decreasing with age, more abruptly from 80 years old. Neither heart disease nor kidney disease, decreased effectiveness.
Conclusion: Although side effects are relatively common, there is no morbidity associated with the high-dose in short cycles of alprostadil, with a considerable effectiveness in not elderly patients and thus, could be considered a useful tool in the treatment of severe non-surgical vascular pathology.
Alprostadil; PGE1; Prostanoid; Ischemic disease; Side effects; Vascular pathology; Medical treatment of ischemia
Prostaglandins are potent vasoactive agents with properties of vasodilatation, fibrinolysis and inhibition of platelet aggregation and in the 80s abundant articles were published that defended the utility of applying these properties in ischemic pathology of the extremities. Althoughalprostadil (PGE1) did not show more efficacy than PGI2, it was used more extensively because PGE1 is easier to administer, it does not require buffering to an alkaline pH, is more stable, has a less critical dose rate, and has less serious and more easily managed side effects [1].
The routes of PGE1 infusion may be either Intravenous (IV) or Intra-Arterial (IA). The IV route needs an increased PGE1 dosage to achieve the same to the arterial route effectiveness, as 90% of PGE1 undergoes metabolic degradation by the first passage from the lung parenchyma. Nevertheless since 1987, PGE1 has been used predominantly intravenously due to potentially serious complications of the intra-arterial route [2].
Abundant articles were published in the 1980s on the efficacy in peripheral arterial disease (PAD) in the 1980s.But in the latest systematic review on PAD published by the Cochrane Lybrary, it is concluded that if the studies with a high probability of bias are eliminated, the risk/benefit balance of the use of prostanoids -without specification of type- in patients with critical ischemia without possibility of revascularization is not clear [3].
However the debate remains open due to the great variability of therapeutic regimens in doses and length in time. Those who in recent years continue to defend the efficacy of the treatment, interpret the lack of positive results in other studies due to the low doses used or the short duration of the treatment [4].
In daily practice it continues to be a therapeutic tool in patients not suitable for revascularization and also as an adjunct when there is residual ischemia after revascularization in PAD [5]. It has also has been used supposedly with success in other ischemic pathologies of the extremities like vasospastic disease associated or not with collagen disease [6] or Buerger’s disease [7].
Most widespread current treatment with PGE1 according to manufacturer recommendation (Altan Pharmaceuticals, Madrid, Spain) and medical publication [8] consists of large periodsthat make treatment somehow cumbersome, either because of the necessary infrastructure for outpatient treatment or because of the long hospital stays that it requires, when the previous option is no available. This regimen is detailed in Figure 1A.
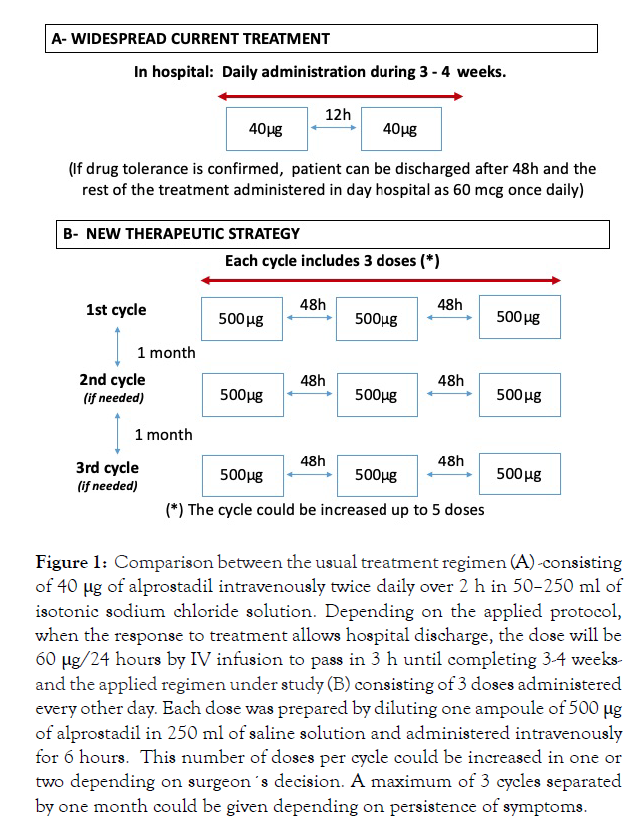
Figure 1: Comparison between the usual treatment regimen (A) -consisting of 40 μg of alprostadil intravenously twice daily over 2 h in 50–250 ml of isotonic sodium chloride solution. Depending on the applied protocol, when the response to treatment allows hospital discharge, the dose will be 60 μg/24 hours by IV infusion to pass in 3 h until completing 3-4 weeksand the applied regimen under study (B) consisting of 3 doses administered every other day. Each dose was prepared by diluting one ampoule of 500 μg of alprostadil in 250 ml of saline solution and administered intravenously for 6 hours. This number of doses per cycle could be increased in one or two depending on surgeon´s decision. A maximum of 3 cycles separated by one month could be given depending on persistence of symptoms.
Background
Our experience in a tertiary hospital, using PGE1 began in 1995. At this time in our country the available presentation was in ampoules of 1 ml containing 0.5 mg (Upjohn ltd).
After an exhaustive revision of the literature an experimental study was developed in minipigs, demonstrating the effectiveness of one high single dose of PGE1 in the studied pathology [9]. Therefore, it was adapted for patients in an innovative regimen with similar doses over time with an intermittent pattern.
A retrospective, observational descriptive study was designed and included all patients treated with this regimen of alprostadil from 2013 to 2020 in a tertiary hospital. Electronic prescribing was implemented in our service in 2013 and for this reason, we could only include patients treated with this regimen since this year.
Therapeutic strategy: New therapeutic strategy shown in Figure 1B.
Variables evaluated: Age, gender, cardiovascular risks factors as diabetes mellitus, hyperlipidemia, hypertension, nicotine abuse, heart or kidney disease were registered and obtained from medical records.
The localization of the symptoms, the presence of ulcer in the symptomatic limb, the number of cycles of medication administered in each episode and the number of episodes treated in each patient as well patients switched to a low-dose regimen were evaluated.
Outcomes: Indications for treatment were registered from medical records.
To evaluate tolerance and effectiveness, adverse events and drug discontinuation, were registered as well as possible medical complications, hospital readmissions within one month, other therapeutic procedures, limb salvage and late survival.
For patients treated in subsequent episodes, only the first episode was assessed for the main analysis. Episode is defined as each independent clinical presentation or reappearance of previous one that had been resolved.
We define Major side effects as those that force to discontinue the infusion and Minor side effects as those that are tolerated with or without a decrease in infusion rate.
Effectiveness was presumed when the medication was tolerated; the treated limb was saved with or without minor amputation, with disappearance of signs and symptoms at rest, without requiring revascularization surgery and without requiring prolonged treatment with an associated regimen of low-dose alprostadil.
Statistical analysis: IBM SPSS statistics software was used for statistical analysis. Categorical variables were expressed as frequencies and percentages while numeric variables where expressed as means ± Standard Deviation (SD). Numeric variables with non-normal distribution were presented by their median.
The possible association of the different variables with effectiveness or appearance of side effects was studied with Fisher's exact test.
Statistical significance was assumed at P <0.05; Patient survival was analysed using Kaplan-Meier methodology. The study was approved by the Ethics Committee for Clinical Research of the hospital.
Fifty episodes were treated in 45 patients, 29 men, 16 women aged between 27 and 91. Two episodes were treated in 2 patients and four in 1 patient.
Patient’s baseline characteristics are shown in Table 1.
| Patients Characteristics | N |
|---|---|
| Age (years) | 62.1 ± 15.16 |
| >75 years | 10 (22) |
| Female | 16 (46) |
| Hypertension | 27 (62) |
| Hyperlipidemia | 23 (53) |
| Diabetes Mellitus | 19 (44) |
| Nicotine abuse | 31 (69) |
| Heart disease | 11 (24) |
| Chronic kidney disease (non terminal) | 5 (11) |
| End-stage renal disease | 2 (4) |
| Data are presented as n (%) or mean ± stand deviation (SD) | |
Table 1: Demographic characteristics and risk factors.
The indication of treatment was ischemia in one of the pathogenic conditions listed in Table 2.
| n (%) | |
|---|---|
| Critical ischemia with macrovascular pathology and bad prognosis revascularization surgery | 5 (11) |
| Critical ischemia with macrovascular disease with no surgical option | 6 (13) |
| Microangiopathy disease with symptoms at rest of acute or chronic evolution | 13 (29) |
| Vascular acrosyndromes and vasospastic disease with symptoms at rest of acute evolution | 4 (9) |
| Complementary after partial revascularization surgery and persistent signs of ischemia | 13 (29) |
| Atheroembolism | 4 (9) |
| Total | 45 (100) |
| Data are presented as n (%) | |
Table 2: Indication of treatment.
Ten patients of 45 presented ulcers in the affected limb (22.2%).
The location of symptoms are shown in Table 3.
| Localization | n (%) |
|---|---|
| Right lower limb | 23 (51) |
| Left lower limb | 15 (33) |
| Both lower limbs | 3 (7) |
| Right upper limb | 3 (7) |
| Left upper limb | 1 (2) |
| The most frequent location of symptoms was the right lower limb | |
Table 3: Location of the ischemia.
Although the treatment protocol is designed with 3-dose per cycle, 3 patients have received 4 doses and 3 up to 5 doses in one cycle.
Twenty nine patients (64%) completed a cycle of 3 or more doses in the first cycle; the mean dose was 2.60; median 3.
Five patients (11%) required more than one cycle: two patients (4%) were treated with 2 cycles and three (7%) with 3 cycles in one episode.
Seven patients (16%) switched to a long low-dose regime, 3 patients without completing cycle and 4 after 3 to 6 doses of high-dose regime.
Total doses per patient are shown in Table 4.
| Doses | n (%) |
|---|---|
| 1 | 9 (20) |
| 2 | 7 (16) |
| 3 | 19 (42) |
| 4 | 3 (7) |
| 5 | 2 (4) |
| 6 | 2 (4) |
| 9 | 2 (4) |
| >9 | 1 (2) |
| Total | 45 (100) |
| Total doses per patient ranged from 1 to 11 mean 3.8; median 3. | |
Table 4: Total doses per patient for the episode.
Safety: Resumed in Table 5. Thirty one patients (69%) did not experience any side effects with good tolerance to the protocol infusion rate, except one who had preventive slowdown for being 91 years old.
| n (%) | |
|---|---|
| Only minor | 8 (17.8) |
| Only mayor | 1 (2.2) |
| Both mayor and minor | 5 (22.5) |
| Total | 14 (31) |
Table 5: Side effects.
Major side effects: Six patients (13%) had serious side effects that required discontinuation of treatment. Half of them during the first dose infusion and the other half along the second one. Five of the six had mild side effects too (significant correlation with p=0.025) None of the patients with drug intolerance reached the third dose.
The causes of suspension were impaired renal function, hypotension, nausea and headache, and phlebitis that did not improve decreasing infusion rate. Association with suspension of treatment was found with heart disease patients in whom 4 out of 11 did not tolerate the medication (p=0.025), and advanced age (in five of ten patients older than 75 years old the treatment was suspended. (p=0.001)
Minor side effects: In 13 patients (29 %) mild side effects were registered, requiring a decrease in the infusion rate when not suspended if associated with mayor effects. Local phlebitis with erythema along the vein and/or local pain in the arm was recorded in 11 patients (24.4%). In all cases, this symptom disappeared immediately at the end of the infusion. Other mild side effects were asymptomatic bradycardia or hypotension, malaise, nausea, flushing and dizziness. The need to decrease the infusion rate because of mild side effects was only correlated with age older than 75 years old (p=0.023). No medical complications appeared after discontinuation of treatment and there were no hospital readmissions within one month for comorbidity.
Effectiveness: According to the established criteria, the treatment was considered effective in 24 patients (53.3%).
No association -negative or positive- has been found with any of the registered variables including indication, total dose, risk factors, heart or kidney disease, presence of ulcer or mild side effects; however, a negative association with age was found (Figure 2).
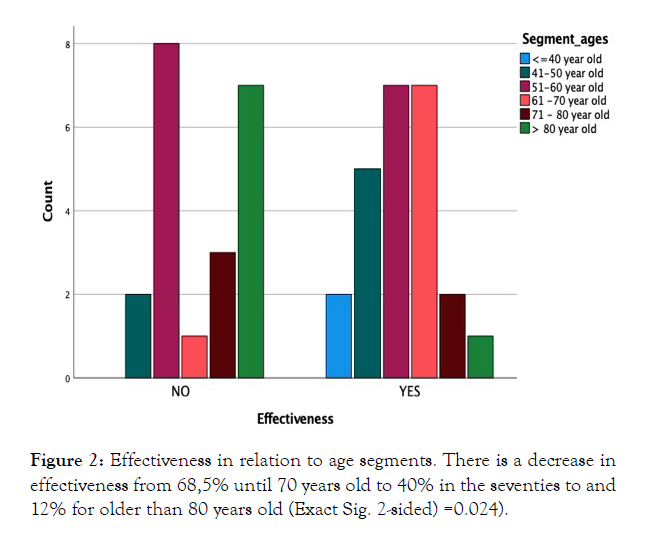
Figure 2: Effectiveness in relation to age segments. There is a decrease in effectiveness from 68,5% until 70 years old to 40% in the seventies to and 12% for older than 80 years old (Exact Sig. 2-sided) =0.024).
Other findings: Of the 16 patients who received just 1 or 2 doses, six cases (37.5%) were because of suspension for intolerance and one because treatment was switched to long treatment at low doses regimen. In nine patients (56%) it was enough to achieve the desired effect.
Limb salvage was achieved in 84.4% patients. Four with associated minor amputation. No association was found with indications, age, ulcer, suspension of treatment, or the presence or absence of risk factors.
Heart disease was no significantly associated with death, nor with effectiveness although it associated with age more than 75 (p=0.007), presence of ulcers (p=0.007) and suspension (p=0.025), as was previously highlighted.
Follow up: Follow up ranged from 3 to 78 months.
Survival curve can be seen in Figure 3.
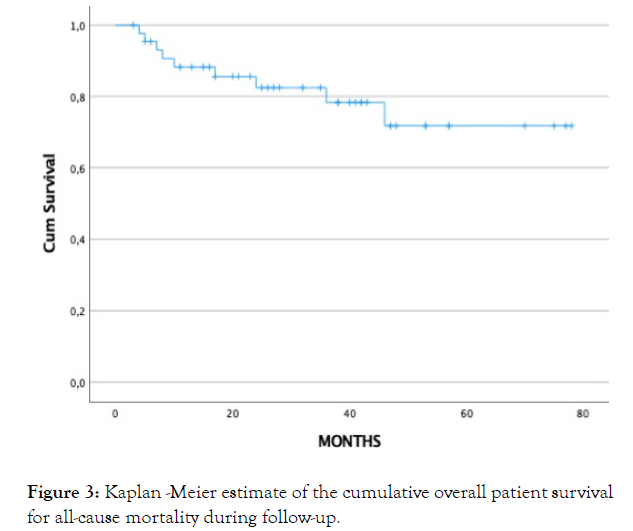
Figure 3: Kaplan -Meier estimate of the cumulative overall patient survival for all-cause mortality during follow-up.
Nine patients (20%) from 58 to 87 years (mean 72.9, SD 12.3) have died along the follow up from 4 to 46 months after treatment with a mean of 17.4 months.
Causes of death are specified in Table 6.
| Cause of death | Nº patients |
|---|---|
| Unknown | 1 |
| Ischemic stroke | 1 |
| Pneumonia | 1 |
| Sepsis limb related | 1 |
| Cardiac failure | 2 |
| Postoperative of unrelated surgery | 2 |
| Unrelated Sepsis | 1 |
| Total | 9 |
Table 6: Cause of death.
A significative association for death was found with being older than 80y (p=0.039) and suffering from kidney disease (p=0.022); both variables are associated between them (p=0.019); (Figure 4).
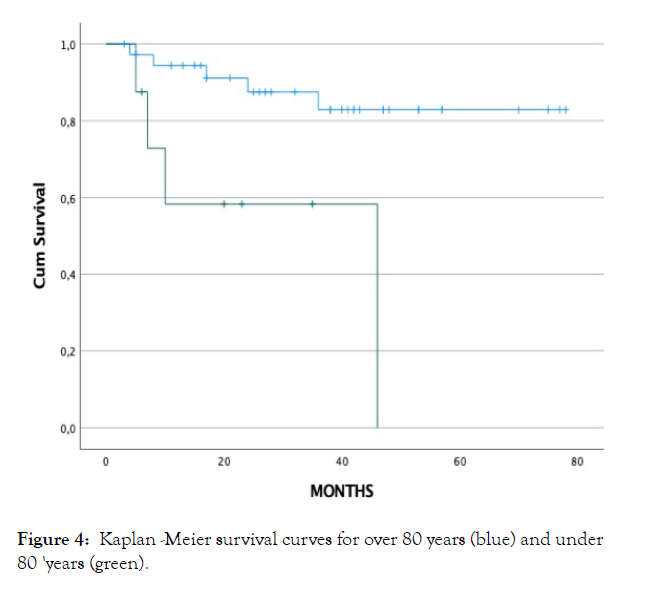
Figure 4: Kaplan -Meier survival curves for over 80 years (blue) and under 80 'years (green).
The first clinical studies with PGE1 were carried out in the 1960s by infusing healthy volunteers with doses of 0.2 mg/kg/min [10].
Carlson et al., [11] reported that doses of 0.1 mg/kg/min (100 ng/ kg/min) in healthy adults caused bothersome side effects such as headache, facial flushing, abdominal cramps, and nausea. To avoid these effects, the efficacy of pGE1 was studied at a lower dose by the intra-arterial route.
In the academic thesis - carried out in our department of experimental surgery - to determine the efficacy of a single dose of alprostadil for the treatment of ischemia produced by the intra-arterial injection of thiopental, one of the difficulties mentioned regarding the experimental model, was to establish the dose to be infused due to the variability found in the literature. Finally, 30 ng/kg/min was chosen, which was prepared by dissolving a 0.5 mg ampoule in 250 ml of physiological serum to be passed in 3h. In this experimental model, the usefulness of a single dose in this indication was demonstrated [9].
For the adaptation to the administration protocol in patients in the department of Vascular Surgery of our centre, we relied on the concept of the highest tolerable dose [12], due to the aforementioned metabolic characteristics and we adopted intermittent infusion - unlike the continuous infusion of most of the works published at the time [13] based on the demonstrated persistence and activity of metabolites for a longer time [14]. Intermittent infusion later became generalized due to the development of tolerance and the rebound effect of platelets in continuous infusion treatments [15].
In our environment, in years after the application of this protocol, a new pharmaceutical presentation of alprostadil emerged in the form of 20 μg ampoules with the long-term dosage recommended by the pharmaceutical companies and the Spanish Medicines Agency (CIMA). This dosage - which we will call standard - is the one used in the largest and most recent randomized, multicenter controlled study designed to assess the efficacy of the medicament in Fontaine stage IVPAD [8]. In this study, which shows no difference between the treated group and the placebo, two findings stand out: the good results in both groups and the high percentage of adverse effects in both groups: 53.8% in the groups treated with alprostadil and the 48.5% in the control group. In our study, 31% patients suffered side effects, more than half of them were mild. In Lawall's study, treatment was suspended due to adverse effects in 5.5% of patients treated with alprostadil and in 6.4% in the placebo group. In our series, 13% of the total required suspension of the medication due to significant side effects, a higher percentage that could be attributed to the drug itself. It is important to note that in all of them the recovery was complete after the suspension; in these patients the treatment has been considered ineffective.
Regarding the surprising good results in Lawall's placebo group, Cronenwett had already observed that one of the important contributions of the studies he analyzed was the demonstration of a high positive response rate, between 40% and 60%, in the placebo´s groups [16]. For this reason, our result in effectiveness must be interpreted with caution, as well as those published in the last decade with standard therapeutic dosage [17]. Rather, we want to highlight the range of indications in which the treatment has been considered indicated from our series; the tolerability of this treatment protocol even in elderly patients, although the increase in adverse effects and especially the decrease in effectiveness makes it prudent not to indicate it in these patients; reversibility of adverse effects including peripheral vein infusion phlebitis; saving in hospital bed when there is no possibility of outpatient treatment; reducing the saturation of health services for the administration of this drug, comfort for the patient, avoiding daily visits to a hospital for 3-4 weeks at daytime.
The clinical situation is evaluated every month, before indicating the next cycle that is only administered in case of persistence of symptoms, which also adds to the saving of resources. In the standard regimen the treatment is applied for 3-4 weeks in any case.
Current evidence does not resolve the question of whether or not there is benefit depending on the doses or if there is benefit in indications other than those studied in multicentre studies. The fact that more than 40 years later the role of alprostadil in limb ischemia continues to be discussed indicates that it possibly has a place where these two variables (doses and indications) must be defined; In our analysis, it has been seen that alprostadil at high doses has been indicated in our hospital in ischemia of the extremities of various origins and evolutionary times. When 20 μg ampoules were available, the standard protocol was also introduced, choosing one or the other depending on the pathology and the professional's preferences, without having carried out comparative studies between the two to date.
Our study has several limitations. First, it is a retrospective study, so one of the most relevant limitations is the difficulty of validating the information obtained from medical records, as it may either be wrong or may not have been recorded. Another limitation is the small sample size. However in our experience, the improvement in certain indications in the first and sometimes only cycle and even with a single dose of 500 μg is very striking and continues to play a role, albeit secondary, in the therapeutic arsenal of the angiologist and vascular surgeon.
Juan Francisco Sedano for his technical assistance
Citation: Cervera T, Ligero JM, Lorenzo A (2022) Safety and Effectiveness of a Shortened Regimen of Alprostadil (PGE1) with Large Infusion Dose, for Ischemic Manifestations of the Extremities in Real-World Clinical Practice. Angiol Open Access. 10:299.
Received: 01-Apr-2022, Manuscript No. AOA-21-10181; Editor assigned: 04-Apr-2022, Pre QC No. AOA-21-10181 (PQ); Reviewed: 18-Apr-2022, QC No. AOA-21-10181; Revised: 25-Apr-2022, Manuscript No. AOA-21-10181 (R); Published: 02-May-2022 , DOI: 10.35248/2329-9495.22.10.299
Copyright: © 2022 Cervera T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.