
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2022)Volume 12, Issue 6
Objective: Percutaneous Coronary Intervention (PCI) for diffuse coronary lesions is challenging because it is difficult to cover all lesions with one stent. Until now, overlapping stents were used to treat diffuse coronary lesions. However, they carry a higher risk of stent thrombosis. In recent years, 48-mm everolimus-eluting stents, which are longer than the conventional stents, have become available, but their safety and efficacy have not yet been established. The aim of this study was to compare the clinical results of a single 48-mm everolimus-eluting stent with those of overlapping stents.
Methods: Between June 2018 and September 2020, 130 consecutive patients with 139 lesions underwent PCI with a single 48-mm everolimus-eluting stent (48S group) or ≥2 Overlapping Stents (OS group). The primary endpoints were adverse events (cardiac death, non-fatal myocardial infarction, target lesion revascularization, and in-stent restenosis). The secondary endpoints were contrast volume, total procedure time, and radiation dose.
Results: The 48S and OS groups had 45 lesions in 44 patients and 94 lesions in 86 patients, respectively. The risk of adverse outcomes was compared using propensity score analysis with 1:1 matching. Kaplan–Meier analysis revealed no significant differences between the groups in relation to adverse events: cardiac death (0% vs. 2.3%; p=0.34), non- fatal myocardial infarction (0% vs. 4.7%; p=0.18), target lesion revascularization (3.4% vs. 3.4%; p=0.96), and in-stent restenosis (4.4% vs. 20.0%; p=0.10). Procedures in the 48S group required less contrast volume (140 (100, 169) vs. 160 (115, 213) ml; p=0.04), a shorter total procedure time (70 (60, 90) vs. 80 (63, 110) min; p<0.05), and lower radiation dose (1.98 (1.46, 3.38) vs. 3.25 (2.12, 4.03) Gy; p<0.01).
Conclusions: The use of the 48-mm everolimus-eluting stent appears to be a safe and effective PCI strategy for diffuse coronary lesions. In comparison with overlapping stents, a very long stent can help simplify PCI procedures.
Coronary artery disease; Percutaneous coronary intervention; Very long stent; Everolimus-eluting stent; Overlapping stents; Diffuse coronary artery disease
The current Percutaneous Coronary Intervention (PCI) with a Drug-Eluting Stent (DES) is associated with a low incidence of stent thrombosis, retreatment, and In-Stent Restenosis (ISR), and its safety and efficacy have been established [1-3]. With the current generation of DES, the occurrence of ISR is low, Target Vessel Revascularization (TVR) occurs in 5.0%-6.3% cases [4], and 10.6% of PCI cases are due to ISR lesions [5]. However, the risk of ISR increases as the DES length increases [6]. In the DES era, stents tend to be longer because it is recommended that stent edges should be placed in the lesion-free area when implanted. Diffuse coronary lesions have a long lesion length; thus, it is difficult to cover all the lesions with one stent, and they are difficult to treat without overlapping stents. However, in the era of first-generation DES, overlapping stents are used in about 10% of PCIs and are associated with impaired long-term clinical and angiographic outcomes compared with those of non-overlapping stents [7]. Further, overlapping stents are associated with a higher incidence of stent thrombosis, cardiac death, Target Lesion Revascularization (TLR), and TVR than single stents in the current generation of DES [8]. In addition, since overlapping stents have a higher fracture rate than do single stents [9], the overlapping segments should be eliminated during stent placement.
Recently, the use of very long stents has been attracting attention to avoid stent overlap. In recent years, 48-mm everolimus-eluting stents (Xience Xpedition, Abbott Vascular, Santa Clara, CA, USA), which are longer than conventional stents, have become available. Diffuse coronary lesions, which until now could only be treated with multiple stents, can now be treated with a single stent. Although there are a few reports examining the safety and efficacy of very long stents, there is a lack of data on their usefulness and safety in clinical outcomes. Therefore, the purpose of this study was to compare the PCI results of a single 48-mm everolimus- eluting stent with those of two or more DES placed at the Nagano Municipal Hospital in the same period.
This was a single-center, retrospective, observational study. Between June 2018 and September 2020, we identified 650 consecutive PCIs and analyzed 139 lesions in 130 consecutive patients who underwent PCI for new diffuse coronary lesions with a single 48- mm everolimus-eluting stent (48S group) or ≥2 Overlapping Stents (OS group). In the 48S group, only single 48-mm everolimus-eluting stents (Xience Xpedition, Abbott Vascular, Santa Clara, CA, USA) were used. In the OS group, 9-40 mm length stents of the current- generation DES, everolimus-eluting stents (Xience Sierra, Abbott Vascular, Santa Clara, CA, USA), platinum-chromium everolimus- eluting stents (SYNERGY, Boston Scientific, Marlborough, MA, USA), cobalt-chromium sirolimus-eluting stents (Ultimaster or Ultimaster Tansei, Terumo Corporation, Tokyo, Japan), cobalt- chromium zotarolimus-eluting stents (Resolute Onyx, Medtronic, Santa Rosa, California), and cobalt-chromium sirolimus-eluting stents (Orsiro, Biotronik AG, Bülach, Switzerland) were used. We excluded patients with multiple stents placed in one vessel but no overlapping stents and those with a 48-mm everolimus- eluting stent with overlapping stents. Additional information on the dates of admission and discharge, sex, age, body mass index, medical history, smoking, examination results, medication status at discharge, and PCI procedure was also collected. Informed consent was obtained in the form of opt-out on the website, and the study was performed in accordance with the Declaration of Helsinki. The study conformed to institutional guidelines and those of the American Physiological Society.
PCI treatment for all patients in this study was performed according to the guidelines of the Japanese Circulation Society [10]. All interventions were performed using standard techniques. The decision of whether to implant a single 48-mm everolimus- eluting stent or ≥2 overlapped stents was based on the operator’s discretion. All patients received Dual-Antiplatelet Therapy (DAPT) with aspirin and a P2Y12 inhibitor (prasugrel or clopidogrel) before PCI and unfractionated heparin during PCI. All patients continued DAPT for 3-12 months according to the guidelines of the Japanese Circulation Society [11]. All interventions were evaluated for lesions by Intravascular Ultrasound (IVUS) or Optical Frequency Domain Imaging (OFDI) before stent implantation. Length of the legion was defined based on IVUS or OFDI findings. Stent expansion and crimping after implantation were also confirmed using IVUS or OFDI.
Overlapping stents were defined as those having overlapping segments of ≥ 1 mm, as determined using IVUS or OFDI. The primary endpoints were a composite of cardiac death, non-fatal Myocardial Infarction (MI), TLR, and ISR. Cardiac death was defined according to the Academic Research Consortium criteria [12]. The definitions of ischemia-driven MI, TLR, and ISR have been published previously [12-14]. The secondary endpoints were contrast volume, total procedure time, and radiation dose.
As the Shapiro–Wilk test revealed that none of the continuous variables were normally distributed, continuous variables are expressed as median values with interquartile ranges (25-75th percentile). Categorical data are expressed as frequencies or percentages. The Mann–Whitney U test was used for continuous variables. For categorical data, the chi-squared and Fisher's exact- tests were used to compare groups. Propensity score analysis with the nearest neighbor matching method was performed. Kaplan– Meier survival plots were created from baseline to the time of an adverse event and compared using the log-rank test. A p value<0.05 was considered statistically significant. All statistical analyses were performed using the EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) and SPSS Statistics version 26 (SPSS, Chicago, IL, USA) software programs.
A total of 174 diffuse coronary lesions in 168 consecutive patients received PCI with 48-mm everolimus-eluting stents or overlapping stents. Thirty-five of these lesions met the exclusion criteria and were therefore excluded from the analysis. The remaining 139 lesions in 130 patients were analyzed (the 48S group had 45 lesions in 44 patients and the OS group had 94 lesions in 86 patients) (Figure 1). All PCI treatments were successful, with a median follow-up of 17.8 months (48S group: 14.2 months, OS group: 19.9 months). In total, 120 patients (92.3%) were followed up for coronary artery by some modality at about one year. Angiographical follow-up was performed for 105 patients; three patients were followed-up by coronary computed-tomography, and 12 patients by myocardial scintigraphy. The remaining patients did not undergo chronic ischemia assessment due to age or financial reasons. The median ischemia evaluation period was 10.3 months (48S group: 9.8 months, OS group: 10.4 months).
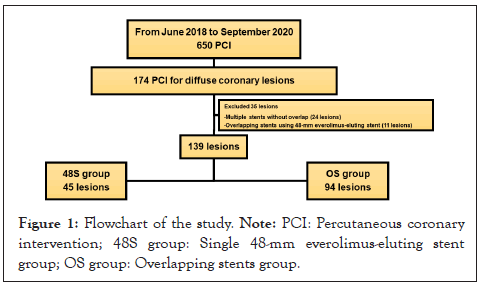
Figure 1: Flowchart of the study. Note: PCI: Percutaneous coronary intervention; 48S group: Single 48-mm everolimus-eluting stent group; OS group: Overlapping stents group.
The baseline clinical characteristics of the patients are listed in Table 1. Their mean age was 72 (67-79) years, and 75.4% of them were male. Patients with diabetes accounted for 33.8% of the sample, patients with multivessel lesions accounted for 73.1%, patients with a history of PCI accounted for 42.3%, and there was a tendency for many patients to have severe coronary atherosclerosis. There were no significant differences between the two groups.
| Total (N=130patients) | 48S group(N=44 patients) | OS group (N=86 patients) | p | |
|---|---|---|---|---|
| Age,years | 72 (67, 79) | 72 (61, 80) | 73 (67, 79) | 0.64 |
| Male,n (%) | 98 (75.4%) | 32 (72.7%) | 66 (76.7%) | 0.62 |
| Bodymass index, kg/m² | 23.9 (21.5, 26.6) | 23.4 (20.9, 26.4) | 24.2 (21.7, 26.8) | 0.41 |
| Hypertension,n (%) | 103 (79.2%) | 36 (81.8%) | 67 (77.9%) | 0.61 |
| Diabetes,n (%) | 44 (33.8%) | 19 (43.2%) | 25 (29.1%) | 0.11 |
| Dyslipidemia,n (%) | 73 (56.2%) | 20 (45.5%) | 53 (61.6%) | 0.08 |
| Dialysis,n (%) | 10 (7.7%) | 2 (4.5%) | 8 (9.3%) | 0.49 |
| Smoking,n (%) | 77 (59.2%) | 28 (63.6%) | 49 (57%) | 0.47 |
| AF,n (%) | 12 (9.2%) | 3 (6.8%) | 9 (10.5%) | 0.37 |
| PAD,n (%) | 28 (21.5%) | 11 (25.0%) | 17 (19.8%) | 0.49 |
| LVEF,% | 62.0 (50.0, 67.0) | 63.0 (50.0, 66.8) | 61.5 (49.8, 67.3) | 0.44 |
| Multivessel,n (%) | 95 (73.1%) | 28 (63.6%) | 67 (77.9%) | 0.08 |
| postPCI, n (%) | 55 (42.3%) | 15 (34.1%) | 40 (46.5%) | 0.18 |
| Aspirin,n(%) | 130 (100%) | 44 (100%) | 86 (100%) | NA |
| Prasugrel,n (%) | 118 (90.8%) | 40 (90.9%) | 78 (90.7%) | 0.62 |
| Clopidogrel,n (%) | 12 (9.2%) | 4 (9.1%) | 8 (9.3%) | 0.62 |
| OAC,n (%) | 12 (9.2%) | 2 (4.5%) | 10 (11.6%) | 0.16 |
| Statin,n (%) | 118 (90.8%) | 38 (86.4%) | 80 (93.0%) | 0.18 |
| Oraldiabetes medicine, n (%) | 40 (30.8%) | 16 (36.4%) | 24 (27.9%) | 0.32 |
| Insulin,n (%) | 7 (5.4%) | 3 (6.8%) | 4 (4.7%) | 0.44 |
| Hb,g/dl | 13.3 (11.9, 14.7) | 13.3 (12.1, 14.6) | 13.4 (11.7, 14.8) | 0.86 |
| HbA1c,% | 6.2 (5.8, 7.1) | 6.2 (5.8, 7.4) | 6.2 (5.8, 7.1) | 0.69 |
| LDLcholesterol, mg/dl | 102 (79, 124) | 107 (79, 132) | 100 (79, 120) | 0.44 |
| TG,mg/dl | 115 (76, 163) | 125 (89, 171) | 110 (71, 161) | 0.28 |
| UA,mg/dl | 5.5 (4.4, 7.0) | 5.5 (4.3, 6.7) | 5.6 (4.5, 7.1) | 0.71 |
| Creatinine,mg/dl | 0.97 (0.80, 1.19) | 0.94 (0.78, 1.11) | 0.99 (0.81, 1.26) | 0.43 |
Note: NA: Not Available
Table 1: Baseline characteristics of patients.
The baseline clinical characteristics of the lesions are listed in Table 2. ST-Elevation Myocardial Infarction (STEMI) accounted for 21.6% of the sample. The target vessels were the left main trunk (LMT) in 12.9% patients, Left Anterior Descending coronary artery (LAD) in 56.1%, Left Circumflex coronary artery (LCX) in 12.9%, Right Coronary Artery (RCA) in 29.5%, and High Lateral branch (HL) in 1.4%. There were 10.1% Chronic Total Occlusions (CTO) and 10.8% true bifurcation lesions requiring the Kissing Balloon Technique (KBT). The lesion length of the target vessel was 48.0 (45.0, 57.0) mm, the total stent length was 48.0 (45.0, 55.0) mm, and the total number of stents was 1.74. The 48S group tended to have more STEMI cases and ad hoc PCI. The OS group had longer lesions, more LMT lesions, more true bifurcation lesions requiring the KBT, and a higher rate of double-lumen microcatheter use. The 48S group required less contrast volume, shorter total procedure duration, and a lower radiation dose.
| Total(N=139 lesions) | 48S group(N=45 lesions) | OS group(N=94 lesions) | p | |
|---|---|---|---|---|
| STEMI, n (%) | 30 (21.6%) | 15 (33.3%) | 15 (16.0%) | 0.02 |
| NSTEMI, n (%) | 8 (5.8%) | 2 (4.4%) | 6 (6.4%) | 0.49 |
| UAP, n (%) | 7 (5.0%) | 4 (8.9%) | 3 (3.2%) | 0.15 |
| Emergency PCI, n (%) | 39 (28.1%) | 17 (37.8%) | 22 (23.4%) | 0.08 |
| Ad hoc PCI, n (%) | 54 (38.8%) | 24 (53.3%) | 30 (31.9%) | 0.02 |
| Target vessel | ||||
| LMT, n (%) | 18 (12.9%) | 1 (2.2%) | 17 (18.1%) | <0.01 |
| LAD, n (%) | 78 (56.1%) | 25 (55.6%) | 53 (56.4%) | 0.93 |
| LCX, n (%) | 18 (12.9%) | 7 (15.6%) | 11 (11.7%) | 0.53 |
| RCA, n (%) | 41 (29.5%) | 12 (26.7%) | 29 (30.9%) | 0.61 |
| HL, n (%) | 2 (1.4%) | 1 (2.2%) | 1 (1.1%) | 0.54 |
| Number of stents, n (%) | ||||
| 1 stent | 45 (32.4%) | 45 (100%) | 0 (0.0%) | NA |
| 2 stents | 85 (61.2%) | 0 (0.0%) | 85 (90.4%) | NA |
| 3 stents | 9 (6.5%) | 0 (0.0%) | 9 (9.6%) | NA |
| Approach site | ||||
| Radial artery, n (%) | 117 (84.2%) | 40 (88.9%) | 77 (81.9%) | 0.29 |
| Distal radial artery, n (%) | 5 (3.6%) | 1 (2.2%) | 4 (4.3%) | 0.48 |
| Brachial artery, n (%) | 3 (2.2%) | 1 (2.2%) | 2 (2.1%) | 0.69 |
| Femoral artery, n (%) | 14 (10.1%) | 3 (6.7%) | 11 (11.7%) | 0.36 |
| Lesion length, mm | 48.0 (45.0, 57.0) | 48.0 (46.6, 48.0) | 51.5 (40.5, 61.0) | 0.01 |
| CTO, n (%) | 14 (10.1%) | 3 (6.7%) | 11 (11.7%) | 0.27 |
| Severe calc, n (%) | 69 (49.6%) | 20 (44.4%) | 49 (52.1%) | 0.4 |
| Kissing balloon technique, n (%) | 15 (10.8%) | 1 (2.2%) | 14 (14.9%) | 0.02 |
| 2-stent technique, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | NA |
| PCPS, n (%) | 2 (1.4%) | 1 (2.2%) | 1 (1.1%) | 0.54 |
| IABP, n (%) | 11 (7.9%) | 1 (2.2%) | 10 (10.6%) | 0.09 |
| Total stent length, mm | 48.0 (45.0, 55.0) | 48.0 (48.0, 48.0) | 55.0 (47.0, 62.0) | <0.01 |
| Guide catheter size | ||||
| 6Fr, n (%) | 102 (73.4%) | 34 (75.6%) | 68 (72.3%) | 0.69 |
| 7Fr, n (%) | 31 (22.3%) | 9 (20.0%) | 22 (23.4%) | 0.65 |
| 8Fr, n (%) | 6 (4.3%) | 2 (4.4%) | 4 (4.3%) | 0.63 |
| Back up guide catheter, n (%) | 80 (57.6%) | 25 (55.6%) | 55 (58.5%) | 0.74 |
| Guide extension catheter, n (%) | 25 (18.0%) | 7 (15.6%) | 18 (19.1%) | 0.61 |
| Micro catheter type | ||||
| Single lumen, n (%) | 89 (64.0%) | 24 (53.3%) | 65 (69.1%) | 0.07 |
| Double lumen, , n (%) | 31 (22.3%) | 4 (8.9%) | 27 (28.7%) | <0.01 |
| Buddy wire, n (%) | 30 (21.6%) | 8 (17.8%) | 22 (23.4%) | 0.45 |
| Pre distal vessel diameter, mm | 3.0 (2.6, 3.8) | 3.0 (2.7, 3.8) | 3.0 (2.5, 3.8) | 0.24 |
| Pre distal lumen diameter, mm | 2.4 (2.1, 2.9) | 2.6 (2.4, 3.0) | 2.4 (2.0, 2.8) | <0.01 |
| Pre proximal vessel diameter, mm | 4.2 (4.0, 5.0) | 4.1 (4.0, 4.9) | 4.2 (3.9, 5.0) | 0.56 |
| Pre proximal lumen diameter, mm | 3.3 (2.8, 3.8) | 3.4 (3.0, 3.7) | 3.3 (2.8, 4.0) | 0.99 |
| Pre reference vessel diameter, mm | 3.3 (3.0, 4.0) | 3.2 (3.0, 4.0) | 3.3 (3.0, 4.0) | 0.76 |
| Pre minimum lumen diameter, mm | 2.0 (1.8, 2.0) | 2.0 (1.8, 2.0) | 2.0 (1.7, 2.0) | 0.83 |
| Pre diameter stenosis, % | 43.3 (33.3, 50.0) | 42.9 (36.2, 50.0) | 43.7 (33.4, 50.0) | 0.75 |
| Pre reference vessel area, mm² | 8.5 (7.1, 12.6) | 8.4 (7.0, 12.6) | 8.6 (7.1, 12.6) | 0.69 |
| Pre minimum lumen area, mm² | 3.1 (2.4, 3.1) | 3.1 (2.4, 3.1) | 3.1 (2.4, 3.1) | 0.74 |
| Pre lumen stenosis, % | 66.7 (56.3, 75.4) | 66.7 (59.5, 75.4) | 66.1 (56.3, 75.4) | 0.64 |
| Post stent minimum lumen diameter, mm | 2.5 (2.3, 3.0) | 2.5 (2.2, 2.9) | 2.5 (2.3, 3.0) | 0.84 |
| Post stent minimum lumen area, mm² | 4.9 (4.0, 7.1) | 4.9 (3.9, 6.8) | 4.9 (4.0, 7.1) | 0.93 |
| Pre dilatation, n (%) | 139 (100%) | 45 (100%) | 94 (100%) | NA |
| Pre dilatation scoring balloon, n (%) | 48 (34.5%) | 17 (37.8%) | 31 (33.0%) | 0.58 |
| Pre dilatation non-compliant balloon, n (%) | 5 (3.6%) | 1 (2.2%) | 4 (4.3%) | 0.48 |
| Pre dilatation balloon minimum diameter, mm | 2.0 (2.0, 2.5) | 2.3 (2.0, 2.5) | 2.0 (1.5, 2.5) | 0.07 |
| Pre dilatation balloon maximum diameter, mm | 2.3 (2.0, 2.5) | 2.3 (2.0, 2.5) | 2.4 (2.0, 2.5) | 0.85 |
| Contrast volume, ml | 160 (115, 200) | 140 (100, 169) | 160 (124, 225) | <0.01 |
| Duration of procedure, min | 88 (65, 120) | 70 (60, 90) | 99 (76, 147) | <0.01 |
| Radiation dose, Gy | 3.01 (1.75, 4.30) | 1.98 (1.46, 3.38) | 3.42 (1.91, 4.78) | <0.01 |
Note: NA: Not Available
Table 2: Baseline characteristics of lesions.
From the baseline characteristics, it is possible that the OS group had more severe coronary lesions, with difficulty in delivering the device due to the longer lesion length, and many bifurcation lesions requiring the KBT in comparison with the 48S group. To eliminate the effect of the differences in the patient backgrounds and lesion characteristics of the two groups, adjustments were made using propensity score matching with the nearest neighbor matching method for each patient and lesion. Propensity score matching identified 45 lesions in 43 patients in each cohort. After propensity score matching, there was no difference in patient background between the two groups (Table 3). Although LMT lesions were still higher in the OS group, the difference between the two groups was less than that before propensity score matching. There was no difference between the two groups with respect to STEMI, ad hoc PCI, and lesion length of the target vessel, KBT, double-lumen microcatheters, and pre distal lumen diameter, which was different before propensity score matching (Table 4).
| Total (N=86 patients) | 48S group (N=43 patients) | OS group (N=43 patients) | p | |
|---|---|---|---|---|
| Age, years | 72 (64, 78) | 72 (60, 80) | 71 (67, 76) | 0.69 |
| Male, n (%) | 67 (77.9%) | 31 (72.1%) | 36 (83.7%) | 0.19 |
| Body mass index, kg/m² | 24.2 (21.7, 26.8) | 23.4 (20.9, 26.4) | 24.6 (21.8, 27.0) | 0.11 |
| Hypertension, n (%) | 69 (80.2%) | 35 (81.4%) | 34 (79.1%) | 0.79 |
| Diabetes, n (%) | 36 (41.9%) | 18 (41.9%) | 18 (41.9%) | 1 |
| Dyslipidemia, n (%) | 47 (54.7%) | 20 (46.5%) | 27 (62.8%) | 0.13 |
| Dialysis, n (%) | 3 (3.4%) | 2 (4.7%) | 1 (2.3%) | 0.5 |
| Smoking, n (%) | 54 (62.8%) | 27 (62.8%) | 27 (62.8%) | 1 |
| AF, n (%) | 7 (8.1%) | 3 (7.0%) | 4 (9.3%) | 0.5 |
| PAD, n (%) | 19 (22.1%) | 11 (25.6%) | 8 (18.6%) | 0.44 |
| LVEF, % | 62.0 (50.0, 66.0) | 64.0 (50.0, 67.0) | 61.0 (49.0, 65.0) | 0.23 |
| Multivessel, n (%) | 60 (69.8%) | 27 (62.8%) | 33 (76.7%) | 0.16 |
| post PCI, n (%) | 36 (41.9%) | 15 (34.9%) | 21 (48.8%) | 0.19 |
| Aspirin, n(%) | 86 (100%) | 43 (100%) | 43 (100%) | NA |
| Prasugrel, n (%) | 78 (90.7%) | 39 (90.7%) | 39 (90.7%) | 0.64 |
| Clopidogrel, n (%) | 8 (9.3%) | 4 (9.3%) | 4 (9.3%) | 0.64 |
| OAC, n (%) | 7 (8.1%) | 2 (4.7%) | 5 (11.6%) | 0.24 |
| Statin, n (%) | 79 (91.9%) | 38 (88.4%) | 41 (95.3%) | 0.22 |
| Oral diabetes medicine, n (%) | 32 (37.2%) | 16 (37.2%) | 16 (37.2%) | 1 |
| Insulin, n (%) | 4 (4.7%) | 3 (7.0%) | 1 (2.3%) | 0.31 |
| Hb, g/dl | 13.7 (12.3, 15.0) | 13.3 (12.0, 14.6) | 13.9 (12.4, 15.6) | 0.26 |
| HbA1c, % | 6.3 (5.8, 7.3) | 6.2 (5.8, 7.4) | 6.3 (5.8, 7.2) | 0.95 |
| LDL cholesterol, mg/dl | 103 (76, 124) | 107 (79, 132) | 102 (76, 120) | 0.39 |
| TG, mg/dl | 119 (81, 172) | 125 (89, 171) | 115 (76, 174) | 0.55 |
| UA, mg/dl | 5.5 (4.3, 6.8) | 5.5 (4.3, 6.5) | 5.5 (4.5, 7.0) | 0.71 |
| Creatinine, mg/dl | 0.93 (0.80, 1.11) | 0.93 (0.77, 1.11) | 0.91 (0.80, 1.12) | 0.99 |
| Cardiac death, n (%) | 1 (1.2%) | 0 (0%) | 1 (2.3%) | 0.5 |
Note: NA: Not Available
Table 3: Baseline characteristics of patients after propensity score matching.
| Total (N=90 lesions) | 48S group (N=45 lesions) | OS group (N=45 lesions) | p | |
|---|---|---|---|---|
| STEMI, n (%) | 25 (27.8%) | 15 (33.3%) | 10 (22.2%) | 0.17 |
| NSTEMI, n (%) | 5 (5.6%) | 2 (4.4%) | 3 (6.7%) | 0.5 |
| UAP, n (%) | 6 (6.7%) | 4 (8.9%) | 2 (4.4%) | 0.4 |
| Emergency PCI, n (%) | 31 (34.4%) | 17 (37.8%) | 14 (31.1%) | 0.33 |
| Ad hoc PCI, n (%) | 41 (45.6%) | 24 (53.3%) | 17 (37.8%) | 0.1 |
| Target vessel | ||||
| LMT, n (%) | 10 (11.1%) | 1 (2.2%) | 9 (20.0%) | <0.01 |
| LAD, n (%) | 52 (57.8%) | 25 (55.6%) | 27 (60.0%) | 0.42 |
| LCX, n (%) | 9 (10.0%) | 7 (15.6%) | 2 (4.4%) | 0.08 |
| RCA, n (%) | 27 (30.0%) | 12 (26.7%) | 15 (33.3%) | 0.49 |
| HL, n (%) | 2 (2.2%) | 1 (2.2%) | 1 (2.2%) | 0.75 |
| Approach site | ||||
| Radial artery, n (%) | 78 (86.7%) | 40 (88.9%) | 38 (84.4%) | 0.54 |
| Distal radial artery, n (%) | 2 (2.2%) | 1 (2.2%) | 1 (2.2%) | 0.75 |
| Brachial artery, n (%) | 3 (3.3%) | 1 (2.2%) | 2 (4.4%) | 0.5 |
| Femoral artery, n (%) | 7 (7.8%) | 3 (6.7%) | 4 (8.9%) | 0.5 |
| Lesion length of target vessel, mm | 48.0 (44.1, 50.6) | 48.0 (46.6, 48.0) | 50.0 (40.0, 60.0) | 0.57 |
| CTO, n (%) | 7 (7.8%) | 3 (6.7%) | 4 (8.9%) | 0.5 |
| Severe calc, n (%) | 44 (48.9%) | 20 (44.4%) | 24 (53.3%) | 0.4 |
| Kissing balloon technique, n (%) | 6 (6.7%) | 1 (2.2%) | 5 (11.1%) | 0.09 |
| 2-stent technique, n (%) | 0 (0%) | 0 (0.0%) | 0 (0%) | NA |
| PCPS, n (%) | 1 (1.1%) | 1 (2.2%) | 0 (0%) | 0.32 |
| IABP, n (%) | 6 (6.7%) | 1 (2.2%) | 5 (11.1%) | 0.09 |
| Guide catheter size | ||||
| 6Fr, n (%) | 70 (77.8%) | 34 (75.6%) | 36 (80.0%) | 0.4 |
| 7Fr, n (%) | 17 (18.9%) | 9 (20.0%) | 8 (17.8%) | 0.79 |
| 8Fr, n (%) | 3 (3.3%) | 2 (4.4%) | 1 (2.2%) | 0.5 |
| Back up guide catheter, n (%) | 46 (51.1%) | 25 (55.6%) | 21 (46.7%) | 0.4 |
| Guide extension catheter, n (%) | 14 (15.6%) | 7 (15.6%) | 7 (15.6%) | 1 |
| Micro catheter type | ||||
| Single lumen, n (%) | 51 (56.7%) | 24 (53.3%) | 27 (60.0%) | 0.52 |
| Double lumen, , n (%) | 13 (14.4%) | 4 (8.9%) | 9 (20.0%) | 0.13 |
| Buddy wire, n (%) | 17 (18.9%) | 8 (17.8%) | 9 (20.0%) | 0.79 |
| Pre distal vessel diameter, mm | 3.2 (2.7, 3.9) | 3.0 (2.7, 3.8) | 3.2 (2.8, 4.0) | 0.74 |
| Pre distal lumen diameter, mm | 2.5 (2.3, 2.9) | 2.6 (2.4, 3.0) | 2.4 (2.0, 2.8) | 0.08 |
| Pre proximal vessel diameter, mm | 4.2 (4.0, 5.0) | 4.1 (4.0, 4.9) | 4.4 (4.0, 5.0) | 0.6 |
| Pre proximal lumen diameter, mm | 3.4 (2.9, 3.8) | 3.4 (3.0, 3.7) | 3.4 (2.8, 4.0) | 0.68 |
| Pre reference vessel diameter, mm | 3.4 (3.0, 4.0) | 3.2 (3.0, 4.0) | 3.5 (3.0, 4.0) | 0.34 |
| Pre minimum lumen diameter, mm | 2.0 (1.8, 2.0) | 2.0 (1.8, 2.0) | 2.0 (1.8, 2.0) | 0.78 |
| Pre diameter stenosis, % | 44.3 (36.5, 50.0) | 42.9 (36.2, 50.0) | 45.0 (37.3, 50.0) | 0.46 |
| Pre reference vessel area, mm² | 9.1 (7.1, 12.6) | 8.4 (7.0, 12.6) | 9.4 (7.1, 12.6) | 0.33 |
| Pre minimum lumen area, mm² | 3.1 (2.5, 3.1) | 3.1 (2.4, 3.1) | 3.1 (2.5, 3.1) | 0.61 |
| Pre lumen stenosis, % | 68.3 (58.1, 75.4) | 66.7 (59.5, 75.4) | 69.6 (56.3, 75.4) | 0.58 |
| Post stent minimum lumen diameter, mm | 2.5 (2.4, 3.0) | 2.5 (2.2, 2.9) | 2.6 (2.4, 3.0) | 0.26 |
| Post stent minimum lumen area, mm² | 5.0 (4.3, 7.1) | 4.9 (3.9, 6.8) | 5.3 (4.5, 7.1) | 0.31 |
| Pre dilatation, n (%) | 90 (100%) | 45 (100%) | 45 (100%) | NA |
| Pre dilatation scoring balloon, n (%) | 34 (37.8%) | 17 (37.8%) | 17 (37.8%) | 1 |
| Pre dilatation non-compliant balloon, n (%) | 3 (3.3%) | 1 (2.2%) | 2 (4.4%) | 0.5 |
| Pre dilatation balloon minimum diameter, mm | 2.3 (2.0, 2.5) | 2.3 (2.0, 2.5) | 2.3 (2.0, 2.5) | 0.87 |
| Pre dilatation balloon maximum diameter, mm | 2.5 (2.0, 2.5) | 2.5 (2.0, 2.5) | 2.3 (2.0, 2.6) | 0.19 |
Note: NA: Not Available
Table 4: Baseline characteristics of lesions after propensity score matching.
The adverse events were cardiac death in 1.2% patients, non-fatal MI in 2.3%, TLR in 4.4% and ISR in 12.2%. ISR was significantly higher in the OS group (4.4% vs. 20.0%; p=0.02) (Table 5). Kaplan– Meier analysis revealed that there were no significant differences in the composite of cardiac death (0% vs. 2.3%; p=0.34), non-fatal MI (0% vs. 4.7%; p=0.18), TLR (3.4% vs. 3.4%; p=0.96), and ISR (4.4% vs. 20.0%); p=0.10) (Figure 2) between the two groups. Even after propensity score matching, procedures in the 48S group required less contrast volume (140 (100, 169) vs. 160 (115, 213) ml; p=0.04), shorter total procedure time (70 (60, 90) vs. 80 (63, 110) min; p <0.05), and lower radiation dose (1.98 (1.46, 3.38) vs. 3.25 (2.12, 4.03) Gy; p<0.01) (Figure 3).
| Total (N=86 patients) | 48S group (N=43 patients) | OS group (N=43 patients) | p | |
|---|---|---|---|---|
| Cardiac death, n (%) | 1 (1.2%) | 0 (0%) | 1 (2.3%) | 0.5 |
| Non-fatal MI, n (%) | 2 (2.3%) | 0 (0%) | 2 (4.7%) | 0.25 |
| Total (N=90 lesions) | 48S group (N=45 lesions) | OS group (N=45 lesions) | p | |
| TLR, n (%) | 4 (4.4%) | 2 (4.4%) | 2 (4.4%) | 1 |
| ISR, n (%) | 11 (12.2%) | 2 (4.4%) | 9 (20.0%) | 0.02 |
Table 5: Major adverse cardiovascular events.
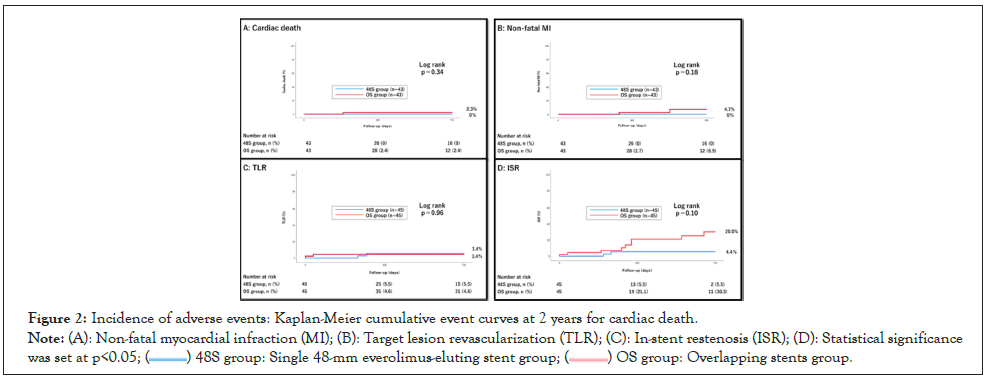
Figure 2: Incidence of adverse events: Kaplan-Meier cumulative event curves at 2 years for cardiac death.
Note: (A): Non-fatal myocardial infraction (MI); (B): Target lesion revascularization (TLR); (C): In-stent restenosis (ISR); (D): Statistical significance was set at p<0.05; ( ) 48S group: Single 48-mm everolimus-eluting stent group; (
) 48S group: Single 48-mm everolimus-eluting stent group; ( ) OS group: Overlapping stents group.
) OS group: Overlapping stents group.
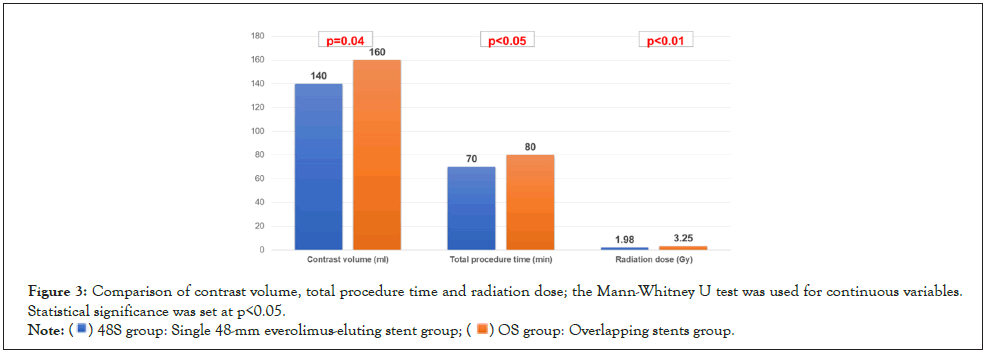
Figure 3: Comparison of contrast volume, total procedure time and radiation dose; the Mann-Whitney U test was used for continuous variables.
Statistical significance was set at p<0.05.
Note: ( ) 48S group: Single 48-mm everolimus-eluting stent group; (
) 48S group: Single 48-mm everolimus-eluting stent group; ( ) OS group: Overlapping stents group.
) OS group: Overlapping stents group.
The main findings of this study can be summarized as follows: (1) the clinical outcome of adverse events was not different between the 48S and OS groups; (2) a single 48 mm everolimus-eluting stent was more beneficial than overlapping stents in terms of contrast volume, total procedure time, and radiation dose. This indicates that a single 48-mm everolimus-eluting stent may be better than overlapping stents in PCI for diffuse coronary lesions.
PCI for diffuse coronary lesions requires not only an increase in the number of stents used but also requires long stent implantation. Longer lesions in PCI have a high risk for ISR [15]. In addition, although overlapping stents are often used during PCI for diffuse coronary lesions, they have limitations. The longer the overlapping segments, the greater the risk of stent thrombosis [8,16]. In addition, ISR is more likely to occur, especially in overlapping segments [7]. The main reason for the frequent ISR in the overlapping segments is considered to be the double-layered stent. Overlapping segments have delayed healing compared to that of non-overlapping segments and promote inflammation at sites of overlap, resulting in uneven concentrations of the drug- eluting on the vessel wall [17]. In addition, overlapping segments may cause stent thrombosis in the acute phase due to lower stent strut coverage within 30 days of PCI [18].
It is necessary to use very long stents for diffuse coronary lesions to avoid overlapping stents. Previous reports have shown that the angiographic and 1-year clinical outcomes of 30 to 38 mm long stents did not differ from those of overlapping stents [19]. The 48-mm everolimus-eluting stent is one of the long stents available in Japan. Its benefits include lower metal content than that in overlapping stent segments and reduction of the risk of stent fracture and geographic miss. However, the safety and efficacy of the 48-mm everolimus-eluting stent are unknown. In recent years, it has shown good one-year clinical outcomes [20, 21]. In addition, for diffuse coronary lesions, the clinical outcome of adverse events between a single 48-mm everolimus-eluting stent and overlapping stents was not different [22]. Additionally, the 48-mm everolimus- eluting stent is more cost-effective [23]. Compared with normal stents, the 48-mm everolimus-eluting stent raises concerns about the difficulty of delivery and risk of stent dislodgement, but the success rate of the procedure is good (97%-100%) [21,24]. In this study, there was no difference in the adverse events associated with the 48-mm everolimus-eluting stent and overlapping stents two years post-operatively, and the procedure success rate was 100%. The incidence of ISR was significantly lower in the 48S group. Although there was no significant difference in the Kaplan–Meier analysis, it tended to be higher in the OS group, demonstrating the safety of the 48-mm everolimus-eluting stent procedure and clinical outcome.
A very long stent can help simplify the PCI procedures. Procedures with long stents reportedly require less contrast volume, shorter total procedure time, and a shorter fluoroscopy time than those with overlapping stents [24]. In our study, the 48-mm, very long stents used significantly less contrast volume. In recent years, the proportion of Chronic Kidney Disease (CKD) patients who receive PCI has been increasing. Patients with CKD have a higher incidence of Contrast-Induced Nephropathy (CIN) than those with normal renal function [25]. The incidence of CIN increases as the maximum allowable contrast dose according to renal function increases [26]. Since cardiovascular events increase in cases of CIN [25,27,28], it is necessary to reduce the contrast volume during PCI as much as possible. The use of a single 48-mm stent may be useful for the prevention of CIN.
Furthermore, in recent years, the radiation dose in medical treatment has become something that cannot be ignored. Procedure radiation exposures, such as during cardiovascular imaging, PCI, and catheter ablation, are reported to account for approximately 40% of all medical exposures, excluding cancer treatment, and have a large impact on patients and healthcare professionals [29,30]. The importance of radiation safety management in medical treatment has been shown in recent years [31]. It is also necessary to minimize the amount of radiation exposure in PCI. The 48-mm stent helps reduce the radiation dose.
As far as we know, this is the first study to compare the clinical outcomes of the single 48-mm everolimus-eluting stent with those of overlapping stents using propensity score matching to align patient backgrounds. The mean follow-up was also longer than that in a previous study, at 17.8 months, and the radiation dose was considered for the first time. In addition, clinically driven TLR was often used as an endpoint in previous studies, but in this study, angiographical follow-up was performed in 80.8% of all cases. Thus, in this study, a more accurate clinical outcome was obtained for TLR.
The study had several limitations. First, it was a retrospective, single- center study with small sample size. A randomized controlled trial is needed to better compare a single long stent and overlapping stents. Second, the choice of stent placement was left to the discretion of the operator and could not be randomized. Third, the lesion backgrounds of both groups were not completely the same. After propensity score matching, the 48S group was more likely to have more simple lesions than those in the OS group, which may have contributed to its clinical outcomes. The inability to compare the two groups equally may have influenced the results. Final, the sample size was relatively small, and the follow-up duration was quite short.
The 48-mm everolimus-eluting stent is a safe device and an effective PCI strategy for diffuse coronary lesions. In comparison with overlapping stents, the long stent can reduce the required contrast volume, total procedure time, and radiation dose, thus effectively simplifying the PCI procedure. In future, accumulation of data on long-term clinical results and cases with difficulty anticipated during stent delivery, such as those of calcified lesions, is necessary.
We appreciate the staff at the Nagano Municipal Hospital for their assistance during the study.
The deidentified participant data will not be shared.
The authors declare that there are no conflicts of interest.
The Institutional Clinical Research Review Boards of Nagano Municipal Hospital (reference no. 2021-11) approved the study protocol.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sunohara D, Miura T, Nomoto F, Itagaki T, Komatsu T, Mochidome T, et al. Safety and Efficacy of a Single 48-mm Everolimus-Eluting Stent in Percutaneous Coronary Intervention for Diffuse Coronary Lesions: A Propensity Score Analysis. J Clin Trials. 12:517.
Received: 24-Oct-2022, Manuscript No. JCTR-22-19813; Editor assigned: 26-Oct-2022, Pre QC No. JCTR-22-19813 (PQ); Reviewed: 09-Nov-2022, QC No. JCTR-22-19813; Revised: 16-Nov-2022, Manuscript No. JCTR-22-19813 (R); Published: 23-Nov-2022 , DOI: 10.35248/2167-0870.22.12.517
Copyright: © 2022 Sunohara D, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.