
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)Volume 11, Issue 3
From the first outbreak in Wuhan (China) in December 2019, until today (05/28/2020), the number of deaths worldwide due to the coronavirus pandemic exceeded 2.5 millions. Only in Argentina, 50,000 deaths have been confirmed so far. There haven’t been so far any clear diagnostic pattern for this exceptional entity except unilateral skin involvement, early onset of symptoms, positive ANA and negative tests for Borrelia burgdorferi. Hence, we report a new case of a young Moroccan man. No treatment tested worldwide has shown unquestionable efficacy in the fight against COVID-19, according to W.H.O, NIH and NICE reports and accumulated data. Our proposal consists of the combination of drugs, based on the pathophysiology of the virus. We have designed a treatment called I.D.E.A., based on four affordable drugs already available on the pharmacopoeia in Argentina, on the following rationale: -Ivermectin (IVM) solution to lower the viral load in all stages of COVID-19. -Dexamethasone 4 mg injection, as anti-inflammatory drug to treat hyperinflammatory reaction to COVID-infection. -Enoxaparin injection as anticoagulant to treat hypercoagulation in severe cases. -Aspirin 250 mg tablets to prevent hypercoagulation in mild and moderate cases. Except for Ivermection oral solution, which was used in a higher dose than approved for parasitosis, all other drugs were used in the already approved dose and indication. Regarding Ivermectin safety, several oral studies have shown it to be safe even when used at daily doses much higher than those approved already. A clinical study has been conducted on COVID-19 patients at Eurnekian Hospital in the Province of Buenos Aires, Argentina. The study protocol and its final outcomes are described in this article. Results were compared with published data and data from patients admitted to the hospital receiving other treatments. None of the patient presenting mild symptoms needed to be hospitalized. Only one patient died (0.59% of all included patients vs. 2.1% overall mortality for the disease in Argentina today, 3.1% of hospitalized patients vs. 26.8% mortality in published data). I.D.E.A. protocol has proved to be a very effective alternative to prevent disease progression of COVID-19 when applied to mild cases, and to decrease mortality in patients at all stages of the disease with a favorable risk-benefit ratio.
In late December 2019, the incidence of atypical pneumonia cases of unknown cause was reported in the Chinese city of Wuhan.
PCR (Polymerase Chain Reaction) studies found a coronavirus, which was >85% similar to a bat SARS-type CoV (bat-SLCoVZC45).
SARS-type CoV (bat-SL-CoVZC45)
The early association identified between SARS-CoV with SARSCoV- 2 was supported by subsequent analyzes, where an important similarity in these transmembrane structures was made clear [1-5].
The only significantly different portion is a furin-binding domain in the SARS-CoV-2 protein S, which may expand tropism or increase virus transmission.
Studies on SARS-CoV proteins have revealed a potential role for IMPα/β1 during infection in the signal-dependent nucleocytoplasmic closure of the SARS-CoV nucleocapsid protein, which may affect host cell division. The predilection and competitiveness of the virus over ACE2 cell receptors has already been demonstrated, and its subsequent need for the importin described above is also confirmed [5-7]. This functional receptor is found in multiple tissues, including alveolar epithelium of the lung, arterial and venous endothelium, smooth muscle, renal tubular epithelium, oropharyngeal mucosa and epithelium of the small intestine, largely explaining the clinical presentation of patients with COVID-19. So has its interaction with TMPRSS2 receptors (Figure 1).
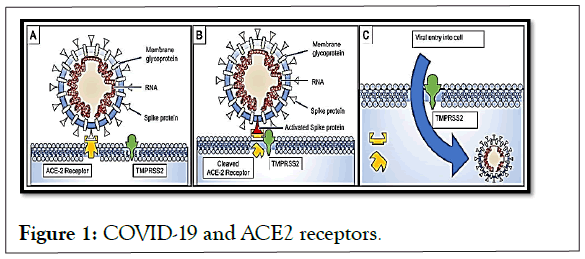
Figure 1: COVID-19 and ACE2 receptors.
Development of clinical events
Before proceeding to list them, we must emphasize two premises.
Between 30% and 50% of patients who contract COVID 19 will be asymptomatic or oligosymptomatic. This fact will not give rise to the consultation, and will directly affect a notorious underregistration of the cases. The second, even more disturbing, premise is that these patients are as contagious as the moderate and severe cases [8-15].
The virus incubation period has been calculated at 5.1 days (95% CI, 4.5 to 5.8 days), and 97.5% of patients are said to have symptoms at 11 days (95% CI 8.2 to 15.6 days). A mortality of 3.1% has been calculated.
The average COVID patient presents with fever (78%), cough (60%-79%) and myalgias or fatigue (35.8% to 44%). 55% develop dyspnea, which appears on average 8 days after the onset of symptoms [16-20].
To the manifestations expressed above, the presence of bilateral conjunctival injection should be added, without associated secretions, hypogeusia, skin rash and hyposmia.
Diagnostic confirmation is made through laboratory studies, which can be performed on a wide variety of biological samples.
Bronchoalveolar lavage samples showed the highest sensitivity (93%), followed by sputum samples (72%), nasal swabs (63%), fiberoptic brush biopsy (46%), pharyngeal swabs (32%), feces (29%) and, finally, blood (1%). A sensitivity of 91% is reported in saliva samples.
Computed Axial Tomography (CAT) is very useful as a complementary study in the diagnostic approach of COVID-19, but this does not discredit follow-up by conventional radiology, if more sophisticated means are not available.
Evidence suggests that a subgroup of patients with severe forms of COVID 19 may have cytokine storm syndrome.
Therefore, we recommend the identification and treatment of hyperinflammation using existing approved therapies with proven safety profiles to address the immediate need to reduce increasing mortality (see therapeutic proposal).
Secondary Hemophagocytic Lymphohistiocytosis (SHLH) is a poorly recognized hyperinflammatory syndrome characterized by fatal and fulminant hypercytokinemia with multiple organ failure.
In adults, SHLH is most often triggered by viral infections, and occurs in 3.7%–4.3% of sepsis cases [21-26].
The cardinal features of sHLH include constant fever, cytopenias, and hyperferritinemia, pulmonary involvement (including ARDS) occurs in approximately 50% of patients.
A cytokine profile that resembles sHLH is associated with the severity of COVID-19 disease, characterized by an increase in Interleukin (IL)-2, IL-7, granulocyte colony stimulating factor, protein 10 inducible by interferon-γ, monocyte chemoattractant protein, macrophage inflammatory protein 1-α and tumor necrosis factor-α.
Mortality predictors from a recent multicenter retrospective study of 150 confirmed cases of COVID-19 in Wuhan, China included elevated ferritin (mean 1297.6 ng/ml in non-survivors versus 614.0 ng/ml in survivors; p<0.001) and IL-6 (p<0.0001), suggesting that mortality could be due to viral hyperinflammation (Figure 2).
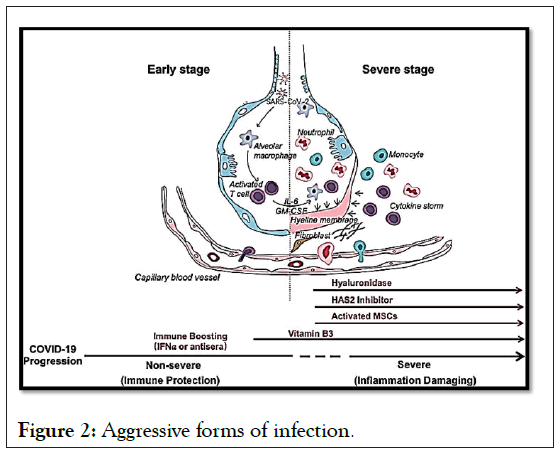
Figure 2: Aggressive forms of infection.
However, cases have been reported where tissue and organ involvement has been found whose concentration of ACE receptors is very dissimilar (myocardium, brain). In all of them, the common denominator was small vessel thrombosis, as seen in entities such as catastrophic antiphospholidic syndrome [27,28].
More than a century ago, Virchow proposed that the formation and spread of thrombi was caused by abnormalities in three key areas.
• Blood flow
• The vascular wall
• The components of blood
These three factors are known as the Virchow triad (Figure 3).
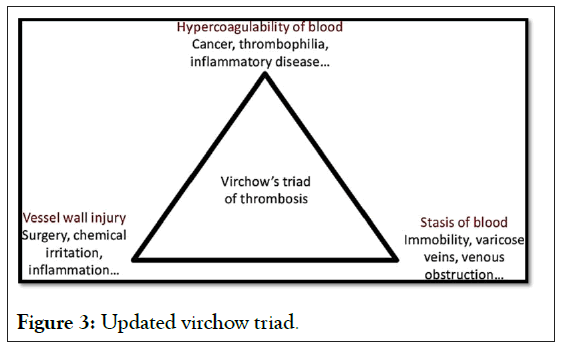
Figure 3: Updated virchow triad.
Currently the Virchow triad factors have been narrowed down in more detail
Circulatory stasis: Abnormalities of hemorrheology and turbulence in vascular bifurcations and stenotic regions.
Injury to the vascular wall: Abnormalities in the endothelium, such as atherosclerosis and associated vascular inflammation.
Hypercoagulable state: Abnormalities in the coagulation and fibrinolytic pathways and in platelet function associated with an increased risk of VTE and other cardiovascular diseases (such as Coronary Artery Disease [CPA], heart failure and stroke in patients with AF). All lead to a state of hypercoagulability, which could explain the formation of microthrombosis in different locations, as has been repeatedly reported in patients with COVID-19 [29,30].
Bases of the proposed therapeutics
They rest on four pillars: Ivermectin, Aspirin, Dexamethasone and Enoxaparin.
Ivermectin
Ivermectin is a broad-spectrum antiparasitic, with vermicidal and ectoparasiticidal properties. It was discovered and marketed for animal use in the early 1980s. Approved in 1997 by the FDA for single-dose strongyllidiasis of 200 mcg/kg and crusted scabies (Scabies Norway) in patients with AIDS at a dose of 200 mcg/kg, every week for 2 weeks [31-34].
In Argentina, it has been available for human use for almost 20 years. But, much more recently, its viricidal effects have been compiled on different varieties of flavivirus, dengue, Zica, Chikunguña, etc.
Ivermectin has been reported to be a SARS-CoV-2 inhibitor. This activity is believed to be due to the dependence of many different RNA viruses on IMPα/β1 during infection. These reports suggested that the inhibitory activity of ivermectin nuclear transport may be effective against SARS-CoV-2, since they demonstrate that ivermectin has antiviral action against the clinical isolate of SARS-CoV-2 in vitro, with a single dose capable to control viral replication in 24-48 hours in vitro.
Apart from the intracell effect of IVM, more mechanisms of action have been described (Figure 4).
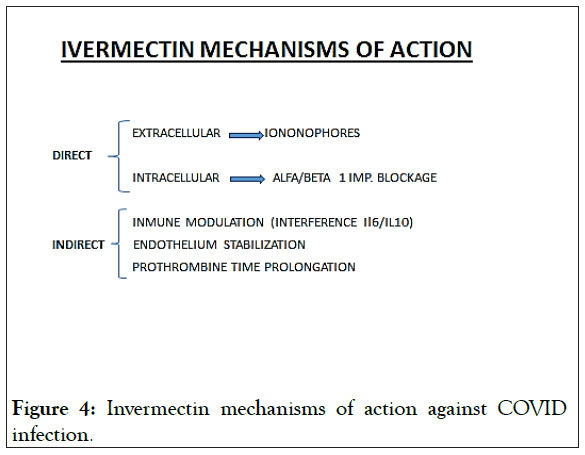
Figure 4: Invermectin mechanisms of action against COVID infection.
Although in vitro studies have used doses that extrapolated to those recommended in the treatment of ectoparasitosis in humans might seem high, the truth is that studies carried out in healthy volunteers, more than two decades ago, proved that the usual doses they can be increased tenfold, without significant side and/or adverse effects.
Aspirin
Aspirin is the common name for acetylsalicylic acid. The chemical production is based on the salicylic acid obtained by synthesis. It’s most common uses and for what it was first used was as an analgesic (for pain), antipyretic (to lower fever) and anti-inflammatory. It is classified as a Non-Steroidal Anti- Inflammatory Drug (NSAID). In 1989 the first large study was published that proved that Aspirin reduces cardiovascular risk, acting as an antiplatelet agent [35].
These were low-dose Aspirin, and the risk of myocardial infarction was found to decrease by 44% when the previously named dose of Aspirin was administered.
Heparin and enoxaparin
Heparins are injectable anticoagulant substances. A distinction should be made between standard Heparin or unfractionated heparin (HNF) and Low Molecular Weight Heparins (LMWH). HNF is made up of a heterogeneous mixture of polysaccharide chains of variable length.
LMWHs are the result of fragmentation of HNF by different methods to achieve products with lower and more homogeneous molecular weights. They are also made up of a mixture of polysaccharide chains and their average molecular weight is much lower. The antithrombotic and anticoagulant activity of HNF is related to the ability to inhibit factor Xa and factor IIa respectively. LMWHs have less inhibitory activity to thrombin or factor IIa but maintain the same potency with respect to factor Xa, so it is expected that they present a lower risk of bleeding but the same antithrombotic activity.
Corticosteroids
Systemic corticosteroids are powerful anti-inflammatory and immunosuppressive agents. They can be administered intravenously, intramuscularly, orally, intralesionally, and topically. Its side effects increase with high, long and frequent doses. Corticosteroids are drugs frequently used in various clinical situations, because they are powerful anti-inflammatories and immunomodulator. Glucocorticoids passively diffuse through the cell membrane, then join soluble receptor proteins in the cytoplasm. They are used, among others, for the treatment of some rheumatic diseases. A separate case, well known but not studied in the current contingency, is acute adrenal insufficiency. The RECOVERY Trial proved DM is useful to reduce mortality (Table 1) [36-38].
| Minor criteria | Major criteria |
|---|---|
| Fever less than 38.5° | Fever higher than 38.5° |
| Isolated diarrheal episodes | Diarrhea (more than 3 diarrheic stools/day) |
| Hyposmia or Hypogeusia | Flictenular conjunctivitis |
| Mild desaturation (between 96% and 93%) | Strong desaturation (less than 92%) |
| Dyspnea without matter | Tachypnea (FR>25/minute) |
| Polymyoarthralgias | Livedo reticularis |
| Persistent headache | Giant urticharia |
| Abdominal pain |
Table 1: The recovery trial proved dm.
I.D.E.A. trial
Based on the precedent data, we performed a Clinical Trial based on the above-mentioned four drugs, on a gradual scale, and according the severity of each case. That Trial, and its outcomes, was duly submitted to the National Library of Medicine (USA) in July 2020. It consisted in a prospective, twoarms (not randomized but based on consentments), study. The control group received all the other options available (hydroxicloroquine, lopinavir-ritonavir, convalescent plasma, etc.). In order to determine the dose and combination, we developed our own severity score (Table 2) [39,40].
| Mild cases | Moderate cases | Severe cases |
|---|---|---|
| Only minor criteria findings | 3 major crit. findings, or 2 major+2 minor | 4 major crit. Findings or 3 major+2 minor |
Table 2: Interpretation.
Equivalences
Dose of 300 micrograms/kg orally, 0.6% drops=1.5 drops/kg of weight
Dose of 400 micrograms/kg orally, 0.6% drops=2.25 drops/kg of weight
Dose of 600 micrograms/kg orally, 0.6% drops=3 drops/kg of weight
When possible, we preferred to use IVM solution instead of tablets, because of its easier absorption and the velocity to reach Tmax and tissue concentration.
Solution is also a better alternative if gastric cannulae shall be used (Table 3).
| Disease severity | Ivermectin | Corticoid | Ventilation | |
|---|---|---|---|---|
| Firm suspicious case or confirmed case | 24 mg orally at a dose of 200 ug/kg in a single dose, to be repeated a week later | No | Aspirin 250 mg orally | No |
| Moderate clinical stage | 36 mg orally at a dose of 400 ug/kg in a single dose, to be repeated a week later | Dexamethasone 4 mg (parenteral) | idem | Low flow washed oxygen or oxygen concentrator |
| Severe case with bilateral pneumonia | 48 mg via gastric cannulae, at a dose of 600 ug/kg in a single dose, to be repeated a week later | idem | Enoxaparin 100 UI/kg (1 mg/kg) | Mechanical ventilation |
Table 3: Disease severity.
Outcomes
According to our clinical criteria, patients were divided into two major groups, outpatients and admitted patients.
Among the admitted patients (Figure 5), death rate was 7 times less with the I.D.E.A. protocol, if compared to all other applied treatments.
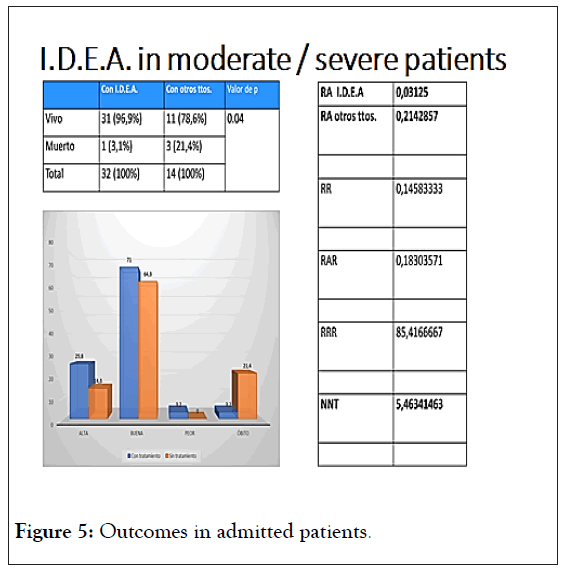
Figure 5: Outcomes in admitted patients.
In this case, it is important to mention that corticosteroids and or enoxaparin were used on both arms.
It is important to emphasize that death was taken as final “adverse reaction”, thus avoiding any subjective bias [41-43].
In the outpatients´group, the adverse effect considered was the need of ulterior admittance, as it has been observed worldwide that this happens in up to 10% of the mild cases, during the two weeks posterior to diagnosis. None of those 135 patients (0.0 %) needed to be admitted (Figure 6).
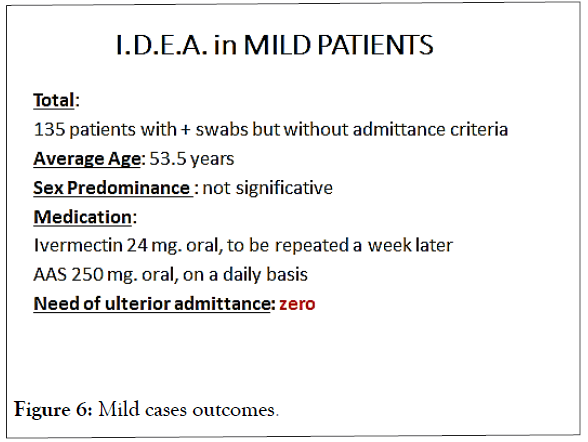
Figure 6: Mild cases outcomes.
This trial received no public or private funding.
The Authors manifest no conflict of interests.
Citation: Hector C, Roberto H (2021) Safety and Efficacy of the Combined Use of Ivermectin, Dexamethasone, Enoxaparin and Aspirina against COVID-19 the I.D.E.A. Protocol. J Clin Trials. 11:459.
Received: 16-Feb-2021 Accepted: 02-Mar-2021 Published: 09-Mar-2021 , DOI: 10.35248/2167-0870.21.11.459
Copyright: © 2021 Hector C, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.