Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2021)
Background: Despite the increasing usage of black cumin (Nigella sativa) oil as dietary supplements/Nutraceuticals, systematic investigations on the role of its major bioactive component thymoquinone (TQ) on safety aspects have not been reported so far. Method: Herein, we report the safety of BCO containing various concentrations of TQ (0.6% and 5% w/w) by single dose acute and 90 days oral toxicity studies in Wistar rats, as per OECD guidelines. Results: While BCO with 0.6% (w/w) of TQ content was safe at 300 to 2000 mg/kg b.wt. upon single dose oral acute toxicity study, LD50 cut off dosage for BCO with 5% TQ (BCO-5) was in the range 50-300 mg/kg b. wt. Further repeated dose 90 days study at 0.1, 0.05 and 0.01 mL/kg of BCO-5 (i.e., at 5, 2.5 and 1 mg of TQ/kg b.wt.) established its NOAEL (no observed adverse effect level) as 0.1 mL/kg (5 mg of TQ/kg b.wt.). Conclusion: Black cumin oil containing 5% (w/w) of TQ content established its NOAEL as 0.1 mL/kg body weight (94 mg/kg b.wt.) which corresponds to 5 mg of TQ per kg b.wt. in rodents and at a level not exceeding 50 mg of TQ/adult/day in humans.
Black seed; Black cumin oil; Dietary supplement; Nigella Sativa; Nutraceutical; Repeated-dose; Safety; Thymoquinone; Toxicity
Nigella sativa, commonly known as ‘black cumin’ or ‘black seed’ has received a recent interest owing to its health beneficial pharmacological activities as evident from the preclinical and clinical studies. In the field of Nutraceuticals, black cumin oil (BCO) is emerging as a miracle herbal agent for health and wellness. According to the American Botanical Council's herbal supplement market research, the sales of black cumin containing products in the USA increased by 200% in 2017 [1]. Black cumin is mainly growing in the Mediterranean region, Middle East, India and Pakistan [2,3]. The plant is mainly cultivated for its seed, and is an approved food flavouring agent and kitchen spice in USA and Europe (FDA 182.20; EFSA 8014-13-9). Seeds and oil of black cumin have been established to possess medicinal effects [2-5]. In the traditional systems of medicine such as Ayurveda and Unani, it is widely using in the treatment of various diseases [5]. Black cumin is known as the 'seed of blessings'. In the Arab systems of medicine, it is also regarded as a ‘cure’ for all forms of diseases [2-5].
Black cumin and its oil have been extensively researched for their therapeutic potential and has been shown to possess broad range of pharmacological activities, including antioxidant, antiinflammatory, antimicrobial, hepatoprotective, anticancer, immunomodulatory, diuretic, antihypertensive, antidiabetic, anthelmintic, gastroprotective, analgesic, spasmolytic, bronchodilator and skin protective effects [6]. Several studies on black cumin and its oil have suggested that the biological activity of BCO could be specifically due to the essential oil components, particularly the thymoquinone (TQ). Several other active compounds, including p-cymene (7-15%), carvacrol (6-12%), 4-terpineol (2-7%), t-anethole (1-4%) and longifene (1-8%) have also been identified as volatile constituents of BCO in addition to the fatty acids such as linoleic acid, oleic acid, palmitic acid and linolenic acid [7].
Several clinical trials of black cumin oil at different dosage ranging from 1 to 12 mL/day have been reported [8]. While most of these studies have not provided an estimate of TQ levels in the oil used in the studies, it has been found that higher oil doses (5 to 10 mL/day) is generally required to provide significant health benefits, suggesting the need for a higher dose of TQ per day. Thus, black cumin oil with higher levels of TQ has recently been widely using as Dietary supplements and Nutraceuticals. However, most of the currently available supplements do not have standardised TQ levels on the label. Furthermore, no systematic studies have been performed on the role TQ content on the safety of these oils. Al- Journal of Nutrition & Food Sciences Safety Assessment of a Thymoquinone-rich Black Cumin Oil (BlaQmax®): Acute and Sub-chronic Toxicity Studies oral acute toxicity study, LD50 cut off dose for BCO-5 was in the range >50-300 mg/kg b. wt. Further repeated-dose Ali et al. have reported the oral LD50 of synthetic thymoquinone as 794.3 mg/kg b. wt. in mice; however no details of this study are available in the public domain, except an abstract [9].Dollah et al. stated that the supplementation of black cumin seed powder at 1 g/kg b. wt. did not produce major changes in liver and kidney function markers [10]. Yet another study by Zaoui et al. reported with no indication of the TQ content in the tested oil [11].
The present study, therefore, attempted to investigate the safety of standardised black cumin oils containing varying TQ levels [0.6 and 5% (w/w)] prepared by a proprietary process of cold pressed extraction technique. In the typical protocol, oils containing varying levels of TQ were first subjected to single-dose acute toxicity study (14 days) to select their safe dose. The selected dosage of highest TQ containing oil was then employed for subchronic (90-day) repeated oral toxicity study to establish the ‘no-observed-adverse-effect-level’ (NOAEL) for future clinical interventions.
Acquisition of materials
Black cumin oil containing 0.6% (BCO-0.6) and 5% TQ (BCO-5) were prepared by a proprietary process of cold-pressed extraction from Indian black seeds and were provided by M/s Akay Natural Ingredients, Cochin, India. Thymoquinone (TQ) content was measured by high-performance liquid chromatography (HPLC) method employing a photodiode array (PDA) detector at 254 nm [12].
Experimental animals
Colony inbred strains of adult Wistar rats of both sex weighing 200 to 250 g were purchased from M/s Nagarjuna Herbal Concentrates Limited, Kerala, India. The animals were housed at the animal house facility of Department of Pharmaceutical Sciences, Mahatma Gandhi University, Kottayam, India in properly ventilated polypropylene cages under controlled temperature (22 to 25°C), relative humidity (60 to 70%) and the light-dark cycle of 12 h. Standard rat feed and water were given ad libitum. One week before testing, the animals were acclimatised to the laboratory conditions and the study was performed as per the protocol accepted by the Institutional Animal Ethics Committee (IAEC). Approval No: MGU/DPS/IAEC/ 2016/PD-5.
Toxicity studies
Schematic representation of the protocol used in the present toxicity studies is depicted in Figure 1. The study was based on the guidelines set by the Organization of Economic Co-operation and Development (OECD). OECD 423 guidelines are employed for single dose acute and OECD 408 protocol was employed for subchronic (90-day) repeated dose toxicity studies [13,14].
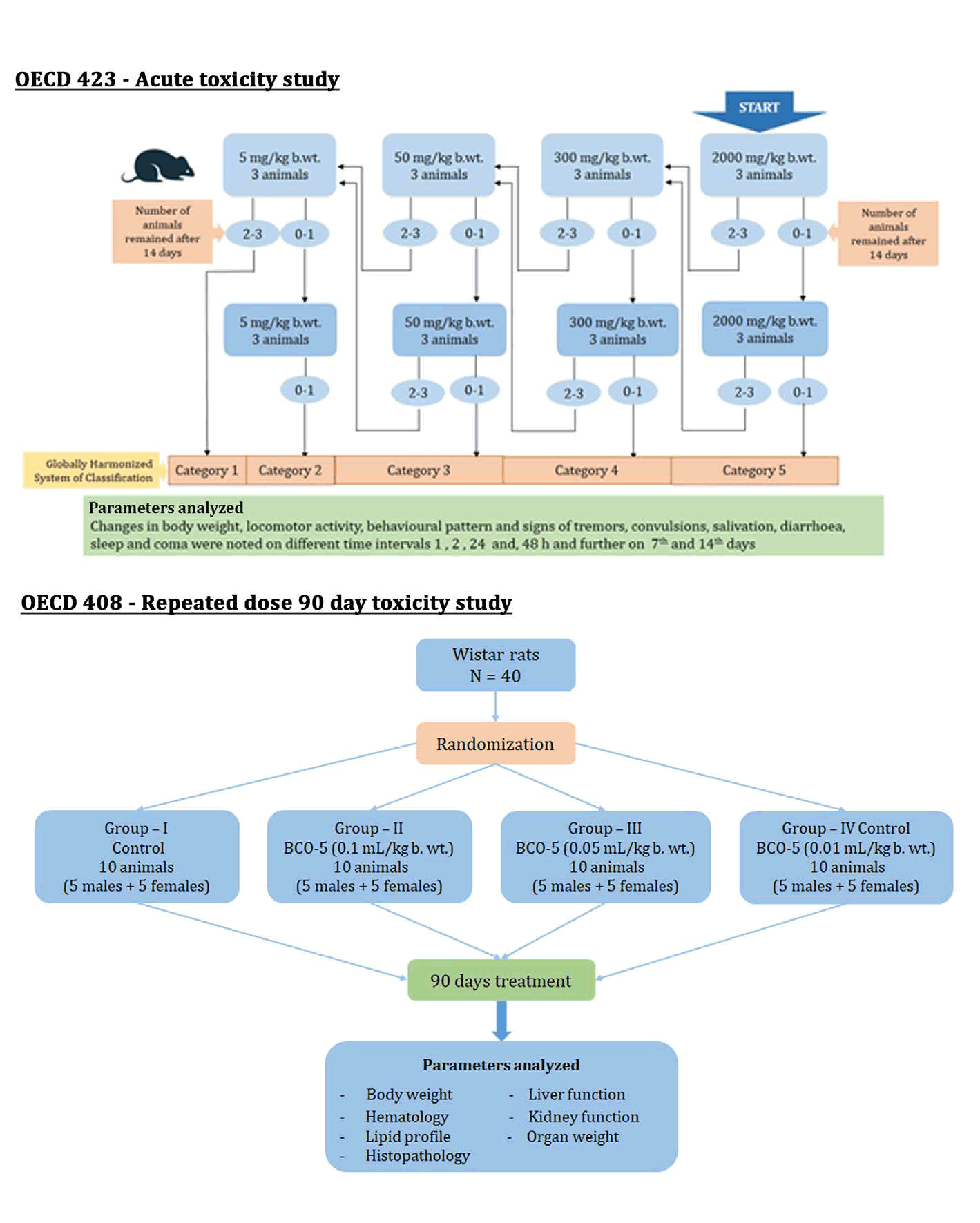
Figure 1: Schematic representation of protocols used in the study.
Acute toxicity study: The method involved a stepwise procedure with 3 animals of single-sex per group with defined doses of 5, 50, 300 and 2000 mg/kg of b. wt. [13]. Adult female Wistar rats weighing 200 to 250 g were used in the present study with a starting dose of 2000 mg/kg b. wt. Changes in body weight, locomotor activity, behavioural pattern and signs of tremors, convulsions, salivation, diarrhoea, sleep, and coma were noted on different time intervals 1, 2, 24 and, 48 h and further on 7th and 14th days. Any signs of adverse effects or toxicity were also noted during the same period.
Subchronic (90-day) repeated dose toxicity study: In the typical protocol, Wistar rats were randomised into three groups containing 5 males and 5 females per each group. Experimental groups G II, G III and G IV were fed with BCO-5 at three dose levels as shown in Table 1 and GI-the control group- was provided with the vehicle. The dosage of the drug was based on the outcomes of acute toxicity trials, and the quantity of drugs administered was decided on the basis of their body weight. Drugs were administered p.o. at the prescribed dose once daily using an oral catheter and continued for 90 days. During this period, the rats were monitored for any adverse reactions and mortality. Body weight and the intake of food/water were recorded. Blood samples were obtained for biochemical experiments on day 91 and the animals were sacrificed under anaesthesia by cervical dislocation. The organs were examined visibly for any type of abnormalities and were excised and weighed. The vital organs like liver, kidney, brain, and spleen were stored in formalin solution for histopathological studies.
| Groups | Dose of oil in mL/kg b.wt. | Dose of oil in mg/kg b.wt. | Dose based on Thymoquinone content (mg/kg b.wt.) |
|---|---|---|---|
| Group I | Control | Control | Control |
| Group II | 0.1 | 94 | 5 |
| Group III | 0.05 | 47 | 2.5 |
| Group IV | 0.01 | 9.4 | 0.5 |
| Note: The dose in mg/kg was calculated based on the density of black cumin oil containing 5% thymoquinone. | |||
Table 1: Dose levels of BCO-5 on animal groups.
Blood biochemistry
The blood was collected by retro-orbital puncture method and was stored in EDTA and non-EDTA vials for assaying haematological parameters and serum biochemistry. Investigated haematological parameters included Haemoglobin, Red blood cell (RBC) count, White blood cell (WBC) count and Platelet, and the serum biochemical parameters included Serum glutamic oxaloacetic transaminase (SGOT), Serum glutamic pyruvic transaminase (SGPT), Alkaline phosphatase (ALP), Albumin, Globulins, Total Protein, Total Bilirubin, Serum Urea, Creatinine, Cholesterol, High-density lipoproteins (HDL), Triglycerides, Low-density lipoproteins (LDL), Very low-density lipoprotein (VLDL) and Serum electrolytes. All the above analysis was conducted as per the standard methods [15].
Histopathology
The animals from the control and higher dose group were sacrificed by cervical dislocation under anaesthesia followed by decapitation and the organs like liver, kidney, spleen and brain were carefully removed. The collected samples were washed with ice-cold normal saline and fixed in 10% formal saline (10 mL of formaldehyde in 90 mL of physiological saline). Paraffin-embedded sections were taken (100-μm thickness), processed in alcohol-xylene series, and stained with Haematoxylin-Eosin (H&E) dye. The sections were examined microscopically for histopathological changes.
Single dose toxicity
During the study period of 14 days, single-dose oral administration of BCO-0.6 at 2000 mg/kg b. wt. did not cause mortality or any adverse reactions. However, BCO-5 at the same dose caused a reduction in body weight. As per the OECD 423 guidelines, the study was then repeated with a starting dose of 300 mg/kg b. wt. of BCO-5. At this dose, the animals showed neither mortality nor changes in body weight, or any clinical signs of toxicity (Table 2). Repetition of the experiment at the same dose of BCO-5 provided similar results. Thus, LD50 cut off mg/kg b. wt. dose for BCO-5 was categorized as Category 3 (>50-300 mg/kg b. wt.), as per OECD.
| Observations (time) | 1 h | 2 h | 24 h | 48 h | Day 7 | Day 14 |
|---|---|---|---|---|---|---|
| Skin and Fur | N | N | N | N | N | N |
| Eyes | N | N | N | N | N | N |
| Mucous membrane | N | N | N | N | N | N |
| Salivation | N | N | N | N | N | N |
| Lethargy | 2/3 | 2/3 | 1/3 | N | N | N |
| Sleep | N | N | N | N | N | N |
| Coma | Nil | Nil | Nil | Nil | Nil | Nil |
| Convulsions | Nil | Nil | Nil | Nil | Nil | Nil |
| Tremors | Nil | Nil | Nil | Nil | Nil | Nil |
| Diarrhoea | Nil | Nil | Nil | Nil | Nil | Nil |
| Morbidity | Nil | Nil | Nil | Nil | Nil | Nil |
| Mortality | Nil | Nil | Nil | Nil | Nil | Nil |
| Note: N-Normal, 2/3, 1/3 -Observation ratio. | ||||||
Table 2: Observations for single-dose toxicity study (OECD 423) of BCO-5 at 300 mg/kg b.wt.
Subchronic (90-day) repeated dose toxicity study
Administration of BCO-5 at 0.1, 0.05 and 0.01 mL/kg b. wt. (i.e., at 5, 2.5 and 0.5 mg of TQ/kg b. wt.) for 90 days showed no significant (P>0.05) decrease in the body weight or in the food and water consumption among both adult male and female Wistar rats (Figure 2). Similarly, none of the animals produced mortality or major adverse events and clinical signs of toxicity. Necropsy revealed no morphological changes or any gross pathological anomalies in the organs. The organ weight such as liver, kidney, spleen, and brain was also remained normal during the study period (Table 3). The assessment of haematological parameters did not show any significant variations (Table 4). Biochemical parameters of liver function, including the marker enzymes, bilirubin, total protein, albumin, and globulin were found unchanged during the study period (ALP levels showed significant change, but was within the safe limit) (Figure 3). In renal function tests, a dose-dependent change in urea levels was noted within the safe limit (Figure 4). However, no change in the creatinine levels was observed (Figure 4). The examination of lipid profile also showed no changes for total cholesterol and HDL, but a decreasing trend for LDL and VLDL (Figure 5). The triglyceride levels were found to be significantly decreased (Figure 5). The histopathological examination of liver, kidney, brain and spleen also showed no specific abnormal patterns (Figure 6), indicating the safety of BCO-5 at the optimized doses of up to 0.1 mL/kg (94 mg/kg b. wt.) or up to 5 mg/kg b. wt. of TQ, for long-term supplementation.
| - | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Male | ||||
| Liver | 3.08 ± 0.20 | 3.63 ± 0.37ns | 3.38 ± 0.21ns | 3.36 ± 0.25ns |
| Spleen | 0.32 ± 0.18 | 0.39 ± 0.22ns | 0.32 ± 0.01ns | 0.33 ± 0.08ns |
| Brain | 0.58 ± 0.05 | 0.64 ± 0.17ns | 0.64 ± 0.01ns | 0.68 ± 0.09ns |
| Kidney | 0.76 ± 0.08 | 0.79 ± 0.07ns | 0.65 ± 0.13ns | 0.68 ± 0.01ns |
| Female | ||||
| Liver | 3.31 ± 0.24 | 3.78 ± 0.42ns | 3.49 ± 0.37ns | 3.27 ± 0.38ns |
| Spleen | 0.34 ± 0.04 | 0.38 ± 0.06ns | 0.36 ± 0.17ns | 0.35 ± 0.32ns |
| Brain | 0.75 ± 0.16 | 0.78 ± 0.05ns | 0.75 ± 0.10ns | 0.74 ± 0.13ns |
| Kidney | 0.77 ± 0.12 | 0.80 ± 0.05ns | 0.68 ± 0.05ns | 0.66 ± 0.06ns |
| Note: Mean ± SD, n=5, ns-non-significant | ||||
Table 3: Organ weight in g of male and female rats following 90-days repeated dose toxicity study of BCO-5.
| - | Group I | Group II | Group III | Group IV |
| Male groups | ||||
| Hb (g/dL) | 15.7 ± 0.51 | 15.08 ± 1.34ns | 15.08 ± 1.34ns | 14.78 ± 1.02ns |
| RBC (106 /cmm) | 6.38 ± 0.02 | 6.14 ± 0.03ns | 6.16 ± 0.41ns | 5.96 ± 0.25ns |
| Platelet (105/cmm) | 4.32 ± 0.34 | 4.86 ± 0.13ns | 4.30 ± 0.14ns | 4.74 ± 0.18ns |
| Total count | 5400 ± 378.00 | 5260 ± 1003.30ns | 5220 ± 1003.30.30ns | 5660 ± 851.46ns |
| Lymphocyte (%) | 53 ± 6.04 | 57.0 ± 6.18ns | 57.0 ± 6.18ns | 54.6 ± 3.21ns |
| Eosinophils (%) | 8.0 ± 2.03 | 5.8 ± 1.02ns | 6.2 ± 2.06ns | 6.4 ± 2.85ns |
| Female groups | ||||
| Hb (g/dL) | 13.6 ± 0.24 | 14.34 ± 0.12ns | 13.94 ± 0.32ns | 14.20 ± 0.21ns |
| RBC (106/cmm) | 5.62 ± 0.21 | 5.86 ± 0.10ns | 5.68 ± 0.01ns | 5.80 ± 0.04ns |
| Platelet (105/cmm) | 4.54 ± 0.06 | 4.18 ± 0.22ns | 4.72 ± 0.42ns | 4.32 ± 0.09ns |
| Total count | 6400 ± 752.86 | 6500 ± 957.49ns | 7900 ± 724.44** | 6540 ± 237.95ns |
| Lymphocyte (%) | 56.6 ± 2.52 | 59.0 ± 1.23ns | 56.01 ± 4.16ns | 58.2 ± 0.49ns |
| Eosinophils (%) | 5.4 ± 4.07 | 6.2 ± 2.54ns | 6.0 ± 0.09ns | 5.4 ± 1.53ns |
| Note: Mean ± SD, N=5, ns-non-significant, **P<0.01 | ||||
Table 4: Haematological parameters of animals, following 90-day repeated dose toxicity study of BCO-5
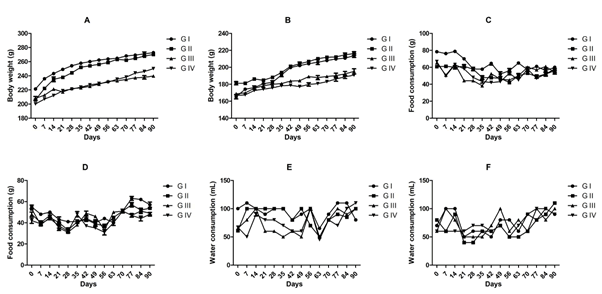
Figure 2: Body weight, food, and wate r consumption on 90-days toxicity studies of BCO-5. (A) Average body weight of male rats (n=5), (B) Average body weight of female rats (n=5), (C) Average daily food consumption of male rats (n=5), (D) Average daily food consumption of female rats (n=5), (E) Average daily water consumption of male rats (n=5), (F) Average daily water consumption of female rats (n=5).
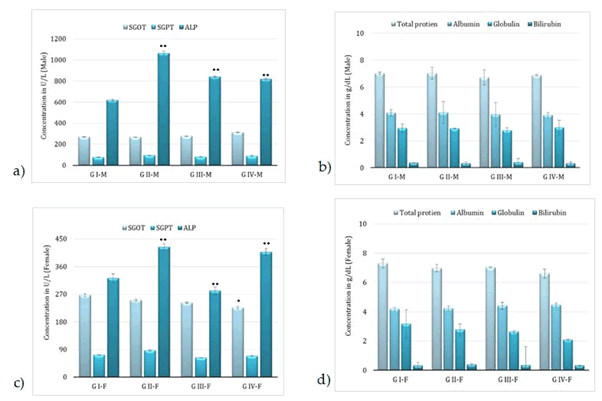
Figure 3: Liver function markers of animals, following 90-days of toxicity studies of BCO-5 – (a) & (b)–male rats and (c) & (d) – female rats (Mean ± SD, n=5, *- P<0.05, **-P<0.001).
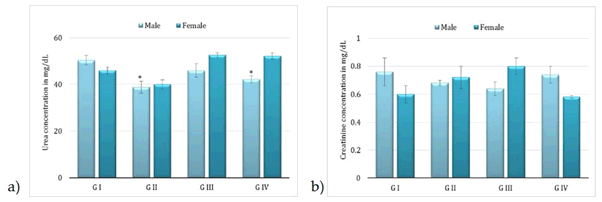
Figure 4: Kidney function tests following 90-days studies of BCO-5. a) Serum urea and b) serum creatinine (Mean ± SD, n=5, * - P<0.01).
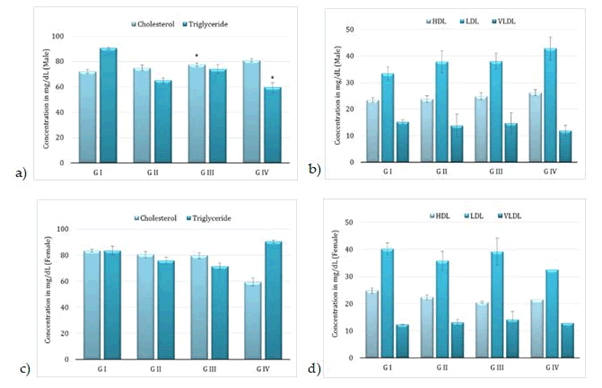
Figure 5: Lipid profile of following 90-days of toxicity studies of BCO-5 – (a) & (b)–male rats and (c) & (d)–female rats (Mean ± SD, n=5, *-P<0.001) Cholesterol (mg/dL), Triglyceride (mg/dL), HDL (mg/dL), LDL (mg/dL) and VLDL (mg/dL)
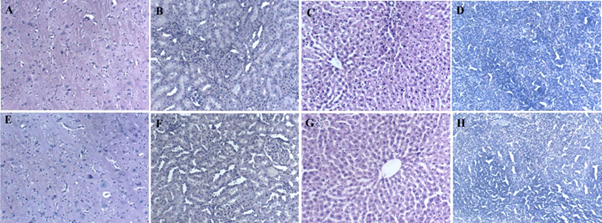
Figure 6: Histopathological results of 90 days of repeated-dose toxicity study (0.1 mL/kg) of BCO-5 by H&E staining. (A) Brain control (B) Kidney control (C) Liver control (D) Spleen control (E) Brain treated (F) Kidney treated (G) Liver treated (H) Spleen treated.
It has been shown that black cumin oil commonly produced by coldpressing or supercritical extraction methods possess a wide range of pharmacological effects and therapeutic potential [6,16]. Many Nutraceuticals/Dietary supplements of black cumin oil produced by cold-pressing techniques and supercritical extraction are already available in the global marketplace. Though dosage ranging from 0.5 to 6 mL/day of black cumin oil was usually suggested by the nutraceutical companies, many of them are not standardized and no information is available on its TQ content. Moreover, no correlation between the TQ content and safety of black cumin oil has been reported to date. Therefore, the objective of the present study was to investigate the safety of black cumin oil as a function of TQ content.
International organizations like OECD and ICH have postulated several guidelines for the safety assessment of drugs and chemicals of both natural and chemical origin. Primarily, OECD 423 guidelines are most used for preliminary testing of drugs, which is a single-dose study. In the present study, black cumin oil with 0.6 and 5% TQ content (BCO-0.6 and BCO-5) were screened at various doses as per OECD guidelines, and found that both BCO-0.6 and BCO-5 are safe and produced no adverse events or mortality at 300-2000 mg/kg b. wt. of oil. However, BCO-5 was found to cause a significant body weight loss. Therefore, further studies at >50-300 mg/kg b. wt. dosage were carried out with BCO-5 and was found to be extremely safe with no abnormalities. In other words, the preliminary test as per OECD 423 showed a dependency of safety on TQ content of BCO in rodents. Previous acute toxicity studies on TQ also had reported a dose-dependent decrease in GSH levels, hypoactivity, respiratory difficulty and even mortality [17].
Subchronic (90-day) repeated dose study was then performed as per OECD 408 guidelines. At dosage of 0.1, 0.05 and 0.01 mL of BCO-5 per kg b. wt., none of the animals showed any specific change in any of the tested parameters including body weight, food/water consumption, haematology, biochemical, organ weight or histopathology, indicating its safety at the optimised dosage of 0.1 mL/kg b. wt. in rats. In terms of milligram weight of oil, the tested dosage is equivalent to 94, 47 and 9.4 mg of oil per kg b. wt. In terms of TQ content, the dosage can be considered as 5, 2.5 and 0.5 mg of TQ per kg b. wt. of animals (Table 1). Since 0.1 mL/kg b. wt. of BCO-5 corresponds to 5 mg/kg b. wt. of TQ, the corresponding safest human dose for clinical interventions or supplementation BCO-5 can be considered at 0.81 mg/kg b. wt./ day of TQ according to the animal dose to human dose conversion [18]. In other words, the safest human dose of BCO-5 for a 60 Kg person may be considered as not more than 900 mg/day.
In summary, the present study demonstrated that the TQ content has a direct influence on the safety of black cumin oil and therefore recommends the standardisation and labelling of TQ content in nutraceuticals containing black cumin oil. While BCO with 0.6 % (w/w) of TQ content (BCO-0.6) was safe at 300-2000 mg/kg b. wt. upon single-dose acute toxicity study, the LD50 cut off dosage for BCO with 5% (w/w) TQ (BCO-5) was found to be in the Category 3 range (>50-300 mg/kg b. wt.) as per OECD guidelines. Further, repeated dose oral toxicity study on BCO-5 established its NOAEL as 0.1 mL/kg b. wt. or 94 mg/kg b. wt. In terms of TQ content, the safe dosage can be deduced as 5 mg of TQ per kg b. wt. in rodents. Upon conversion to human dosage, the safe dosage may be a maximum of 900 mg of BCO-5/adult/day or 48.6 mg of TQ/ adult/day.
Authors KR & SI are thankful for M/s Akay Natural Ingredients, Cochin, India for the well-characterised sample of Black cumin oils used in the present study.
The author(s) declared the following conflicts of interest concerning the authorship and/or publication of the article. KR, SI & RK belongs to the non-profitable institution and has no conflict of interest. SDS & KIM are employees at Akay who manufactured BCO.
Kannan Ramalingam: Investigation, Data curation and Writingoriginal draft preparation. Ramadassan Kuttan: Reviewing and Editing of Manuscript. Syam Das S: Data curation, Writing- Original draft preparation. Krishnakumar IM: Conceptualization, Methodology and Reviewing. Sibi Ittiyavirah: Conceptualization, Methodology, Supervision and Writing- Reviewing and Editing.
Citation: Ramalingam K, Kuttan R, Sivadasan SD, Ittiyavirah S, Madhavamenon KI (2021) Safety Assessment of Thymoquinone rich Black Cumin (Nigella sativa) Oil (BlaQmax®): Acute and Sub chronic Toxicity Studies. J Nutr Food Sci. 11:811.
Received: 14-Jun-2021 Accepted: 28-Jun-2021 Published: 05-Jul-2021
Copyright: © 2021 Ramalingam K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.