Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Review Article - (2021)Volume 11, Issue 5
COVID-19 has caused death and major upheavals worldwide. A zoonotic disease that has escaped its natural animal host and infected humans to cause a global pandemic. It has strained the healthcare capacity of nations ill-prepared to deal with this disease. Vaccination to achieve group immunity is desirable. Restraints are issues of vaccine monopoly by rich nations able to vaccinate most of its population while under-developed nations only a small fraction. Newer variants are likely to emerge. Non pharmacological measures will for the future remain primary preventive measures.
COVID-19; Health systems; Tests for COVID-19; Vaccines; Immunity; Hesitancy challengers
The Municipal Health Commission of Wuhan, China reported an outbreak of pneumonia on 31 December 2019 [1]. On 11 March 2020 the World Health Organization (WHO) alarmed by the human-to-human spread and its severity declared a novel coronavirus disease SARS-CoV-2 a major worldwide pandemic. Termed COVID-19 it caused severe respiratory illness, other major organ dysfunction and death [1].
SARS-COVID-19 has had a major impact on healthcare systems and has challenged human resources of countries worldwide. It is highly infectious and has strained the capacity of health systems resulting in increased mortality and morbidity globally. The global death from the disease is 4.2 million and more than 200 million of the world’s population has been infected with COVID-19 as of 30 July 2021. Advanced economies like the USA have 640 thousand reported deaths since the start of the pandemic. Developing economies like Brazil and India have staggeringly high numbers of around 579000 and 437000 respectively [2].
National economies have been hard hit and may take many years to fully recover. The work space environment has been transformed by work from home with more reliance on social media platforms.
The pandemic has posed major challengers in the lives of health care professionals and frontline workers many have succumbed to the disease and others report worries and anxieties regarding the risks of infection, infecting members of their household, difficulties coping with mental health issues and from dying of the disease [3].
Long term COVID which has emerged as sequelae of the illness continues to impact on the quality of life of sufferers physically, socially and economically [4].
Following the approval of vaccination in states worldwide, this has allowed anxieties to be remedied. Evidence from the SISONKE15 (see below) study suggests that vaccination prevents serious infection, hospitalisation and death. Community and workplace support programs will play a major role in aiding individuals and families in maintaining their mental, emotional and physical wellbeing [3].
This paper will highlight the common tests used in COVID-19 and the challenges relating to their interpretation in the context of the clinical background and disease prevalence in which the illness occurs. It further emphasises the importance of vaccination as a means of achieving herd immunity, the distortion in vaccine distribution worldwide and its consequences.
In addition, it offers comments on the vaccines at the forefront in Europe, US and in particular the SA experience and discusses vaccine hesitancy. Concluding remarks relate to collaboration and future preparedness of all role players (Figure 1).
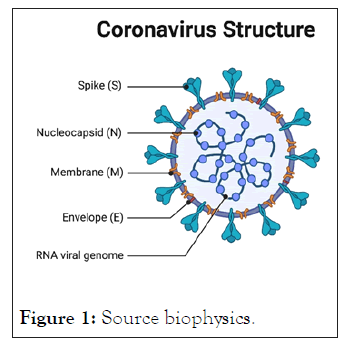
Figure 1: Source biophysics.
Tests for COVID-19 and disease surveillance
The Polymerase Chain Reaction (PCR) test is considered the gold standard by the Centre of Disease Control (CDC) in the USA. Nucleic Acid Amplification Test (NAAT) which detects Viral Nucleic Acid (RNA) is the recommended modality for diagnosing acute SARS-CoV-2 infection [5]. Laboratory based NAAT is the test of choice for diagnosing COVID-19 illnesses.
The test amplifies the virus’s genetic material and requires cotton swabs taken from the nasal or oral-pharynx [6]. A nasal mid-turbinate or anterior nasal swab detects viral RNA and is accurate and efficient in detecting COVID-19 before the person becomes infectious.
The disadvantages include the high costs, results for laboratory processing may take 1-3 days and testing requires trained staff [6]. Some facilities have the capacity to make results available early at the Point Of Care (POC).
COVID Rapid Antigen test is a quick screening tool to detect an acute infection. It requires a nasal or pharyngeal swab and detects viral proteins at the peak of infection. It is less sensitive than the RT-PCR test and infected persons may pass as a false negative test. These become positive with replication of the virus and may not in the early phase of COVID-19 show the presence of the virus [6]. A negative Ag test in patients with symptoms and signs of COVID-19 should be confirmed by a NAAT result.
Rapid Ag test results are known within 15 minutes at the POC; it is less expensive and requires less experienced staff or laboratory testing [7]. It is useful test when there is a high demand and delays in PCR testing.
This test is able to identify specific mutations as variants emerge like the beta and delta variant and can be designed for others likely to emerge [7].
Antibodytest
This is a serological test that requires blood. This test measures antibodies (IgG, IgM) to the CoV-2 virus in persons who have already had COVID-19 infection or who have been successfully vaccinated [7]. Antibodies offer protection and may persist up to 6 months or longer. It is a measure of the immune response mounted against the virus and not used for diagnosis of an acute infection.
Percent positive
This is the percentage of all tests that are actually positive. A high number indicates that infection is more widespread in areas where testing is done. The WHO recommends a figure below 5%. Health officials use this number to tract the pandemic and make recommendations regarding lockdowns and other restrictive measures [8].
R number
R0 reproductive number is the number of people that an infected person can infect. The aim is to have R number below one. If R is greater than 1 the number of new cases will increase. If R equals 1 the number of new cases is stable and if R is less than 1 this implies the number of infected individuals will decrease over a period of time [9].
Case 1: This was an 80-Year-old male, a known hypertensive who had a low-grade fever for 1 week with loss of appetite. Now hypotensive and with early signs of confusion the patient was hospitalized and investigations for COVID-19 begun.
Investigations
PCR test for COVID was negative. Routine tests including a Full Blood Count (FBC), Urea and Electrolytes (U&E) were normal. Inflammatory marker C-Reactive Protein (CRP) and Iron (S-Ferritin) were raised. Plain Chest X-rays (CXR), were followed with a Computer Tomography (CT) scan as well as a Ventilation/Perfusion (V/Q) scan of the lungs (Figures 2 and 3).
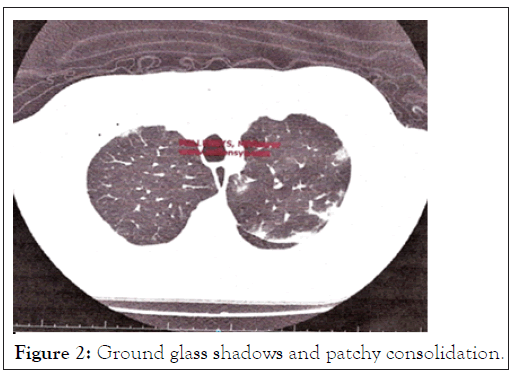
Figure 2: Ground glass shadows and patchy consolidation.
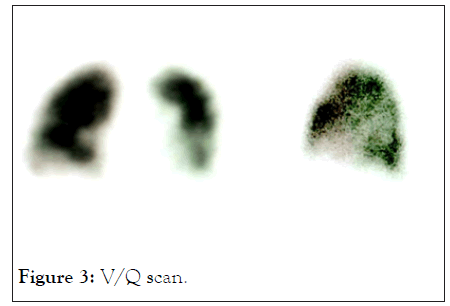
Figure 3: V/Q scan.
Repeat PCR done 3 days later was positive.
On admission
• CRP Patient value 53.6 (Normal value<5.0).
• S-Ferritin Patient value 2132 (Normal 300 n/ml).
• Leukocytes normal count.
• CT of the lungs suggested patchy regions of ground-glass shadowing with patchy consolidation, highly suggestive of COVID-19 (Figure 2).
V/Q scan showed irregular perfusion in areas of the lung bases but no general emboli present in Figure 3, Results were suggestive of stage III and a hyper inflammatory response to the COVID virus [10].
The patient was in stage III in terms of the severity of his illness at admission despite a negative PCR test (Figure 4). He was stabilized with fluid rehydration and given low flow nasal oxygen. He Maintained good oxygen saturation (mid 90s) and was not in any respiratory distress. He became normotensive and his vital signs remained stable.
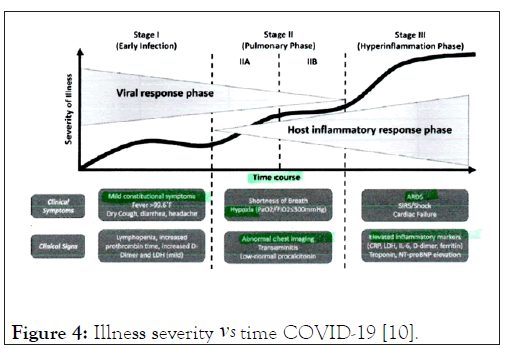
Figure 4: Illness severity vs Time COVID-19 [10].
Treatment included antibiotics, steroids, prophylactic anticoagulants and immune supplements. He remained in isolation for 2 days and was discharged for home care and self-isolation for 10 days.
Implication of false negatives
During the early phase of the pandemic PCR tests were in overwhelming demand with long turnover time of 24 hrs or longer for obtaining results. Rapid antigen testing at the POC was unavailable. Sampling techniques involved taking swabs from the oral-nasal pharynx. Handling errors or inadequate sampling could give a false (-) negative result and consequently risk exposure to COVID-19 transmission to healthcare workers.
The history and clinical impression of the patient were in keeping with COVID-19 illness.
He was isolated and PPE protocols observed. A subsequent PCR test on day 3 confirmed SARS-CoV-2 infection. Personal Protective Equipment (PPE) and adherence to protocol has huge implications for costs. There is also slower turnover time in patient management and precautions taken as breaks in protocol can cause spread of infection.
Case 2: An adult caregiver nursed a parent who had died of COVID-19. She tested negative for a Caovid-19. The nasal swab done was described by the patient as being very painful and unpleasant. She subsequently reported a unilateral nasal discharge and was treated for congestion and sinusitis with antihistamines. Her condition did not settle and on chemical testing of the fluid it was found to be Cerebral Spinal Fluid (CSF). A CT Cisternography of the brain confirmed a leak site through the cribriform plate. Surgery to close the CSF leak was done through a Tran’s nasal approach and the leak successfully sealed (Figures 5 and 6). The delay from the time of insult to the surgical closure was 6 months.
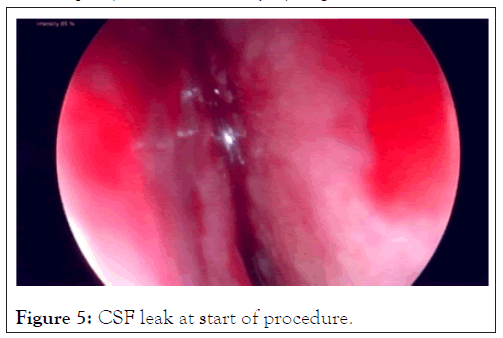
Figure 5: CSF leak at start of procedure.
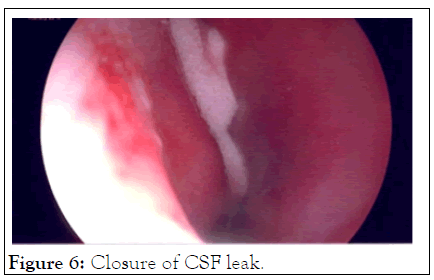
Figure 6: Closure of CSF leak.
This is a rare complication. PCR testing requiring nasal swabs can be done either shallowly or deep and care must be taken not to cause pain, discomfort or injury (Figures 5 and 6).
Case 3: A 74 yr. old male gave a history of melena stools and was scheduled for gastroscopy and colonoscopy. The patient had COVID illness 6 weeks prior and was managed at home with no complications. Treatment was supportive with oxygen, steroids and aspirin. He gave a history of ischemic heart disease and a cardiac assessment showed him to have normal cardiac functions on echocardiogram and no signs of ischemia with exercise but tired very easily. His oxygen saturation was in the low 90% after minimal effort.
His lung functions and CXR were normal. The patient did not exhibit any clinical signs of COVID-19 disease. He was apyrexial with normal vital signs. The overall clinical impression was that his physical decline, dyspnoea, and fatigue were in keeping with post-acute COVID syndrome (long COVID). COVID antigen tested negative but a COVID PCR test was positive.
The positive PCR was likely due to the persistence of viral antigens than any active secretion of the virus. Replication competent SARS-CoV-2 is unlikely after 2 weeks.
Post-acute COVID-19 syndrome is seen as long-term complication of the acute illness beyond 4 weeks from its start. Its effects include fatigue, shortness of breath, chest pain, arthralgia, mental cloudiness and a decline in lifestyle [4]. The endoscopy was done under sedation with standard PPE protocols.
New technologies have been developed since the start of the pandemic and laboratory based NAATS is highly sensitive and specific for diagnosing COVID-19. Other methods using Isothermal amplification are being evaluated namely LAMP (Loop Mediated Isothermal Amplification).
Infective Diseases Society of America (IDSA) offer comprehensive recommendations and guidelines in the diagnosis of COVID-19 and Antigen testing [11].
Considerations are the likelihood of the individual having the illness as well as its prevalence in a community. The context in which testing is being conducted, whether it be screening within a hospital, nursing home, schools or community screening or home environment.
The case selections highlight challenges within a hospital environment when capacity and demand for testing were overwhelmed during the early phase of COVID-19. Handling and laboratory errors can occur to give a false negative test for COVID-19 as shown in case 1. The clinical suspicion suggested covert-19 which was confirmed with a subsequent repeat positive test. Symptomatic patients who initially test negative should receive an additional NAAT as suggested by guidelines [5].
The symptoms of Influenza and flu like illness are not dissimilar to COVID-19. Influenza is a seasonal disease and may occur currently during a pandemic.
Case 2, illustrates a rare complication with sampling techniques and specimen collection.
Nasopharyngeal (NP) swabs for sampling may pose problems. The newer technology molecular tests allow for samples taken from saliva which can be considered comparable to NP swabs for SARS-COV-2 NAAT this is less traumatic and easier to perform [5].
Trained professional staffs are required when deep nasopharyngeal are done and so reduce sampling errors (case 1) and risks of complication (case 2).
Presently home kits for testing are commonplace and may result in other complications like pain, epistaxis and further minor irritations.
Finally, in case 3 a screening rapid Ag test was negative. The individual was asymptomatic and had acute COVID-19 illness 6 weeks earlier. However, his RT-PCR tested positive. In comparing the two tests rapid Ag tests have modest sensitivity and high specificity compared to NAATS. Rapid Ag has sensitivity of 81% in symptomatic persons and 49% in asymptomatic patients, and a specificity of 99% compared to NAATS [11].
The positivity is more likely due to remnants of the virus genetic material being shed as RNA which can be detected long after the risk of transmission has passed [12]. The isolation period is 10 days from the onset of symptoms after which patients are not infectious. The high sensitivity of the laboratory NAATS may pick up low viral load in someone who is not infective or has recovered.
The possibility that the positive result is due to reinfection of COVID-19 is unlikely although reinfection may be possible and can occur as early as 45 days after the first illness [12].
A development is the CDC flu SC2 Multiplex Assay a single test able to diagnose one of three viruses: SARS-COVID-2, influenza A and influenza B. This test for public health monitoring will not replace current COVID-19 diagnostic tests [13].
Case 3, also had features of Long COVID which is now a recognised clinical syndrome with huge bio-psycho-social impact across all age group of the adult population and on all organ systems [4]. Physical and mental decline occurs and particularly in the elderly can lead to social isolation and food insecurity in the absence of family and community support structures. Full recovery may take many months following an acute illness [14].
The ongoing COVID-19 pandemic remains unabated. Developed nations with a sizeable proportion of their population now fully vaccinated are seeing new variants become dominant. These such as the Delta variant are more infectious and deadly then the previous Alpha and Beta variant.
Developing nations with scarce resources have limited access to vaccines despite a global pledge to global equity. Vaccine monopoly and control by developed nations will further fuel the epidemic and create an environment for new variants developing. The solution lies in a more equitable distribution of vaccine to countries with limited resource. Presently less than 2% of low-income countries have been vaccinated and 27% of the world’s population while the developed nations (UK, USA, EU) have vaccinated a sizeable part of their population [15].
Global vaccine front runners
• These are approved in the UK, Europe, US and Canada.
• Oxford-AstraZeneca viral vector type vaccine used largely in the (UK).
• Pfizer-BioNTech (mRNA) vaccine
• Moderna (mRNA) vaccine
• The latter two used in the US and Canada requires very low temperature for storage.
All of the above require a double dose of the vaccine.
Other vaccines include Jansen/(Johnson & Johnson) a single dose vaccine used in South Africa (SA), Sinopharm, Sinovac and many others awaiting licencing by regulatory authorities in countries.
The SISONKE study using the Janssen (J&J) vaccine was given to health workers in SA and completed on 17 May, 2021 [16].
In a report tabled August 5 2021 it concluded [16]:
• The vaccine is safe.
• It is effective against the Delta variant.
• It protects against severe disease and death.
• A good immediate immune response is provided and durable up to 8 months after a single dose.
• Breakthrough infections may occur but its effects are mild.
Vaccine hesitancy and existing challenges
Resistance and pushbacks to vaccination are inevitable. This poses a risk to the individuals themselves and the larger community. Educating the public on vaccination and counteracting misinformation is required. Public officials and private enterprise may need to incentivize and mandate vaccination so individuals can weigh the cost benefits in terms of travel restrictions, recreational opportunities and potentially even employment.
The mainstay in control of the pandemic is vaccination on a global stage aiming at vaccinating most of the world’s population. Herd immunity will occur when most of the population achieves immunity either naturally or through vaccines. This will stop further spread of the virus. The Delta variant is currently a major threat will require vaccination of 84% of a population to achieve herd immunity [16].
Non pharmacological measures will remain part of the primary preventive measure as will Testing for COVID-19, masking, social distancing, hand washing, contact tracing, quarantine and isolation part of the everyday vocabulary in the control of COVID disease. Predictions are that pre COVID-19 normality will be delayed unless and until populations have achieved immunity.
The SARS-COVID-2 pandemic has resulted in the unprecedented worldwide collaboration between governments, scientists, clinicians and other role players. The rapid development of tests to identify the virus, the genetic sequencing of the virus and its variants, the development of vaccines with novel technologies (mRNA vaccine), its fast tracking and deployment, the shared knowledge of therapeutics in the management of COVID-19 illness are all positives and auger well for a world that must be better prepared for future pandemics.
Figures 2 and 3 Dr. Matisonn. Brenthurst Clinic.
Figures 4 and 5 Dr. M R Ahmed.
Layout M Bulbulia BA (Unisa).
Dr. Amina Jakoet Saban Ph. D (UCT) for comments and recommendations.
Y Bulbulia MA (UFT) for reviewing the manuscript.
Author BA B has no conflict of interest to declare.
Citation: Bulbulia BA (2021) SARS COVID-19 Case Studies: Diagnostic Testing and Vaccination Challenges. Intern Med. 11: 348.
Received: 02-Sep-2021 Accepted: 16-Sep-2021 Published: 23-Sep-2021 , DOI: 10.35248/2165-8048.21.11.348
Copyright: © 2021 Bulbulia BA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.