
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2022)Volume 13, Issue 6
Objective: Cell-Mediated Immunity (CMI), which includes T-cells (both T helper and cytotoxic), is critical for effective antiviral defenses against CoronaVirus Disease-2019 (COVID-19). Expression of several CD markers analyzed in few patient groups to better understand immunological properties of CD markers in post-COVID-19 patients.
Materials and methods: Flow cytometer was used to quantify total lymphocyte count and assess expression of CD markers in the samples.
Results: Percentage of Lymphocytes decreased significantly in post-SARS-COV-2 patients compared to normal subjects, a usual expression in any viral infection. In contrast CD8+ population was increased in patient groups with prolonged SARS-COV-2 infection coupled with or without comorbid complications.
Conclusion: These results corroborated with earlier reports of SARS-CoV-2 infection in overall total lymphocyte status while long post-SARS-COV-2 case(s) showed unexplained up regulation of CD8+ subset which needs further elaboration as a possible predictable cellular marker in all long post-SARS-COV-2 cases whether or not multi-organ affection is obvious (cardiac, renal etc.,). Also there may well be a need of extension of time window of post-SARS- COV-2 infection leading to multi-organ affection from current 3-6 months to possibly years even.
Cell-mediated immunity; Post-SARS-COV-2 multi-organ disease; CDs as immune markers; Post-SARS- CoV-2 coronary-cardiac presentations
Coronavirus Disease 2019 (COVID-19) is caused by the latest coronavirus species to infect humans and is characterized by severe acute respiratory symptoms brought on by a novel beta coronavirus known as Severe Acute Respiratory Syndrome CoronaVirus-2 (SARS-CoV-2). SARS-CoV-2 belongs to the Nidovirales virus family, which also includes the Coronaviridae [1]. Symptomatic clinical presentations range in severity from mild-fever and dry cough to severe shortness of breath, fever, and dry cough with blood oxygen saturation dropping below cut-off of around 93% and respiration rate greater than 30 per minute [2]. A specific furin position in spike protein and a mutagenic phylogenetically messy open reading frame1ab (Orf1ab) of SARS-CoV-2 set it apart from other RNA viruses [3]. SARS-CoV-2 virus has a diameter of 60–140 nm and is typically pleomorphic. It is sensitive to UV radiation and heat [4,5]. The key parts of the Entire structure (E) are the Spike (S), the Nucleocapsid (N), the Membrane (M), and the Envelope (E), with a genomic size of 30 kb [6]. It has been documented that SARS-CoV-2 entrance site is the Angiotensin- Converting Enzyme 2 (ACE2), which is present on surface of small intestinal enterocytes, nasal epithelium and lung alveolar epithelial cells among other locations along with tongue and oro-pharynx. Interruption of immune system regulation, increased metabolic demand, direct cardiomyocyte affection and extensive procoagulant activity are believed to be some of the causes of increased risks of poor outcomes in persons with COVID-19-related Cardio-Vascular Disease (CVD). SARS-COV-2 is connected with cardiovascular system through events such as myocardial injury, myocarditis, acute myocardial infarction, heart failure, dysrhythmias, and venous thromboembolic events [7,8]. An attempt was made in the study to compare post-COVID patients with cardiac-coronary presentations to healthy persons in order to assess differential status of CMI.
Interaction of innate and adaptive immune systems in body controls how the host responds to infections, including viral infections. Molecular interactions of T lymphocytes, particularly CD4 and CD8 subsets are essential both for the humoral and cellular immune system to respond to any infections including viral infections [9].
Available data reveals intermediate CD16 LDNs (low-density neutrophils) are associated with illness severity because they are more prevalent in severe patients than in moderate cases. Another CD marker, CD138 is a type I transmembrane proteoglycan that is a member of the syndecan family and consists of a core protein that has been glycosylated with chondroitin and Heparan Sulphate (HS) moieties. Among others, CD138 has been associated with cell adhesion, endocytosis and the Ag migration to nucleus [10]. CD19 is a known marker of B-lymphocyte malignancies including B cell lymphomas, Acute Lymphoblastic Leukaemia (ALL) and Chronic Lymphocytic Leukaemia (CLL). Most B cell malignancies express CD19 Ag at levels ranging from medium to high [11]. CD16 and CD56 is the typical phenotypic marker of natural killer cells. However, it can also be seen in alpha/beta T cells, gamma/delta T cells, dendritic cells, and monocytes [12].
Reagents and materials
CDs markers antibodies with the following catalogue codes were acquired from BD Bioscience, India: CD56 (340363), CD4 (561005), CD16 (560995), CD8 (561952), CD69 (560968), CD45 (340953), CD138 (347193), and CD19 (340409).
Clinical samples
Clinical samples were from Apollo Multispeciality Hospital, Kolkata. Patients were grouped as under:
1. Normal Subjects- (Figure S1 and Table 1).
| Normal Individuals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/Sex | History | Lymphocytes | CD4 | CD8 | CD16 | CD19 | CD69 | CD138 | CD56 | |
| 20-24/M | Healthy individuals with no previous medical history | 13.3% | 28.2% | 36.2% | 18.9% | 6.15% | 0.60% | 0.086% | 21.1% | |
| 20-24/F | 6.33% | 39.8% | 25.8% | 14.6% | 8.12% | 0.30% | 0.12% | 21.6% | ||
Table 1: Represents biomarker expression in normal individuals.
2. Cardiac-Coronary presentation with current SARS-COV-2 infection (Figure S3 and Table 2).
| Active SARS-COV-2 Infection with cardiac problems | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | History | Lymphocytes | CD4 | CD8 | CD16 | CD19 | CD69 | CD138 | CD56 | |
| 65-69/F | Diabetic, the hypertensive female patient presented with shortness of breath for the last 3 days and mild fever for the last 7 days. Significant findings include left ventricular dilatation with severe systolic dysfunction (left ventricular ejection fraction was 30%, and there was grade 2 Mitral regurgitation) on ECHO. The ECG showed atrial fibrillation with a rapid ventricular response. The COVID RT PCR was positive (CT of N gene 25 and RdRP 26.She was treated with anti-arrhythmic drugs, oxygen supplementation, and other supportive medications. She improved and was discharged 2 weeks later in hemodynamically stable condition. | 2.73% | 33.8% | 18.7% | 28.6% | 25.6% | 3.71% | 0.14% | 16.3% | |
Table 2: Immune biomarker expression in individuals with active SARS COV 2 infection with cardiac problems.
3. Cardiac-Coronary presentation with no SARS-COV-2 infection (Figure S4 and Table 3).
| No SARS-COV-2 infection with Cardiac--Coronary presentation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/ Sex | History | Lymphocytes | CD4 | CD8 | CD16 | CD19 | CD69 | CD138 | CD56 | |
| 65-69/M | Presented with intermittent chest pain associated with exertional dyspnoea for the last 6 months, which was progressive. A coronary angiogram revealed double vessel coronary artery disease, for which percutaneous transluminal coronary angioplasty and stenting weredone. The post-procedural period was uneventful. | 12.6% | 28.3% | 54.2% | 24.4% | 2.06% | 1.92% | 0.14% | 15.5% | |
| 60-64M | Hepatitis B positive male presented with chest pain for 2 days. ECG showed non-ST elevated AMI. A coronaryangiogram revealed critical double vessel coronary artery disease. The patient was managed with percutaneous transluminal coronary angioplasty with stenting. Post-procedural period was uneventful. | 4.68% | 19.3% | 15.3% | 51.0% | 12.0% | 2.87% | 0.077% | 47.6% | |
| 65-69/M | Presented with intermittent chest pain associated with exertional dyspnoea for the last 6 months, which was progressive. A coronaryangiogram revealed double vessel coronary artery disease, for which percutaneous transluminal coronary angioplasty and stenting weredone. Post-procedural period was uneventful. | 3.55% | 41.1% | 28.2% | 33.1% | 10.8% | 3.62% | 0.0% | 15.3% | |
Table 3: Represents biomarker expression in individuals without SARS-COV-2 infection with cardiac-coronary presentation.
4. Cardiac-Coronary presentation with recent SARS-COV-2 infection (Figure S5 and Table 4).
| Recent SARS-COV-2 infection WITH Cardiac-Coronary presentation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/ Sex | History | Lymphocytes | CD4 | CD8 | CD16 | CD19 | CD69 | CD138 | CD56 | |
| 70-74/F | Diabetic and hypertensive female admitted in April 2022 with complaints of 2-3 episodes of presyncopeattack associated with dizziness. She had COVID-19 in January 2022, for which she was managed with oxygen supplementation and other supportive medications. ECG done during the present admission showed bradycardia with bifascicular heart block and only minor coronary disease in the angiogram. A permanent pacemaker was implanted, and she has improved after that. | 5.09% | 36.6% | 17.9% | 26.0% | 9.85% | 1.06% | 0.070% | 24.3% | |
| 50-54/M | Complained of chest discomfort in the beginning of April 2022. Echocardiography revealed septal hypokinesia with tema left ventricular dysfunction (Left Ventricular Ejection Fraction was 48%). ECG Shankax showed a left bundle branch block. CT angio was done, which showed 70-80% Occlusion of the left circumflex artery. The patient gave history of COVID-19 in November 2020, for which he has advised home isolation and was treated with Flavipiravir, HCQS, Ivermectin, and vitamins. His saturation was well maintained during his illness with COVID, and he did not require oxygen supplementation for the same. Presently he is being treated for his cardiac problems with beta-blockers and ACE inhibitors |
3.92% | 39.4% | 30.4% | 31.6% | 2.05% | 0.72% | 0.12% | 33.0% | |
Table 4: Represents immune CD marker expression in individuals with recent SARS-COV-2 infection showing cardiac-coronary presentation.
5. Cardiac-Coronary presentation with long SARS-COV-2 infection (Figure S2 and Table 5).
| Prolonged SARS-COV-2 infection WITH Renal presentation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/ Sex | History | Lymphocytes | CD4 | CD8 | CD16 | CD19 | CD69 | CD138 | CD56 | |
| 55-59/M | A non-diabetic, moderately hypertensive male experienced rapid onset chest pain. An ECG taken at the time revealed signs of ischemic heart disease. Ischemic heart disease and grade II diastolic dysfunction were discovered on echocardiography. In June 2020, he was diagnosed with COVID and was treated with hydroxychloroquine, vitamins, and antipyretics. He didn't need oxygen because he was able to sustain oxygen saturation in room air. He is currently on beta-blockers, vasodilators, and anticoagulants. He also takes anti-oxidants. [150mg levo-carnitine + 200mg vitamin E] | 1.52% | 31.9% | 50.1% | 16% | 2.62% | 0.37% | 0% | 16.6% | |
Table 5: Represents immune biomarker expression in individuals with long SARS-COV-2 infection with Cardiac—Coronary presentation.
6. Renal presentation with long SARS-COV-2 infection (Figure S6 and Table 6).
| Prolonged SARS-COV-2 infection WITH renal presentation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/ Sex | History | Lymphocytes | CD4 | CD8 | CD16 | CD19 | CD69 | CD138 | CD56 | |
| 50-54/F | Borderline renal parenchymal disease was admitted to the hospital with fever, anorexia, weakness, body ache, dizziness in September 2020. She was diagnosed withcovid infection. COVID RT PCR was positive with CT value N 25 and RdRP 26). She was treated conservatively and did not require steroids or oxygen. Later she developed chronic kidney disease. (urea 61 mg/dl and creatinine 2.9 mg/dl) | 1.52% | 36.5% | 43.5% | 12.5% | 0.65% | 0.91% | 0.25% | 22.9% | |
Table 6: Represents immune biomarker expression in individuals with long SARS-COV-2 infection with renal presentation.
7. Long SARS-COV-2 infection with No Clinical Presentation (Figure S7 and Table 7).
| Long SARS-COV-2 infection WITH No Clinical Presentation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age/ Sex | History | Lymphocytes | CD4 | CD8 | CD16 | CD19 | CD69 | CD138 | CD56 | |
| 35-39/F | Both the patients were asymptomatic and were inhome isolation. No treatment was received | 4.24% | 48.2% | 26.7% | 12.1% | 5.05% | 0.81% | 0.067% | 19.3% | |
| 35-39/F | 5.65% | 51.6% | 24.0% | 10.5% | 10.7% | 0.40% | 0.00% | 37.3% | ||
Table 7: Represents the immune biomarker expression in individuals with long SARS-COV-2 infection without clinical presentation.
Current post-SARS-COV-2 long COVID time window is defined to be within 3–6 months post primary infection. However, post- COVID cardiac patient reported here had an overt cardiac presentation after 22 months without having any preceding cardiac signs and symptoms even remotely ever. 5 ml blood samples of fresh whole blood were taken in EDTA tubes in each case.
Quantification of CDs markers
FACS tube was filled with 100 μl blood sample. 5 μl of each antibody was added for every 100 μl of sample followed by 15 minutes incubation. Next, 1X lysis buffer (3 ml) was added followed by 10min incubation. Samples were centrifuged for 5 minutes at 1500 rpm. The cell pellet was resuspended in 1X PBS after removal of supernatant. Samples were centrifuged again at 1500 rpm for 5min. Supernatant was discarded and 1X FBS was added. Flow Cytometer (BD LSRII Fortessa) was used to measure CD markers on the collection day and Flowjo software was used to analyze the results.
Clinical characteristics of study subjects
Objective of study was to evaluate quantitative expression of CD markers (mostly CD4 and CD8) in post-COVID-19 coronary and renal presentations compared to that of healthy individuals, primarily. Other clinical subgroups were unique by differential expression of immunological biomarkers and lymphocyte subsets.
Immune biomarker expression: Patients with long-term SARS COV 2 infection and coronary presentation had noticeably higher CD8 levels than other patients. Compared to the typical participants, patients with long-term SARS-COV-2 infection had elevated CD4 counts. Comparing long-term SARS-COV-2 infection patients with renal difficulties to healthy people and those with SARS-COV-2 infection without complications, it was discovered that these patients had lower CD19 levels and higher CD8 levels. A high CD8 population was detected in one patient (TC), who had a cardiac presentation but no clinically obvious SARS-COV-2 infection. Any following extended study needs to be more purposeful about this.
Those with cardiac symptoms who had active SARS-COV-2 had greater CD19 levels than patients in other groups. An elevated level of CD4 and CD8 was seen after a prolonged infection with SARS- COV-2. Active SARS-COV-2 infection increased the level of CD19. The presence of comorbid complications showed variation in levels of immune biomarkers in patients, as shown in Tables 1-7.
Viral infections cause activation of both acquired and innate immune systems. Memory population against viral infection including SARS-COV-2 is the CD4+ and CD8+ stands for cytotoxic subset [13]. Major Histocompatibility Complex (MHC) class I-mediated antigen presentation plays a crucial part in host defense against intracellular infections and cancer. MHC class I promotes antiviral immunity improving the way viral antigens are presented to CD8+ cells. Virus-infected cells are destroyed preferentially by activated CD8+ cytotoxic T cells [14].
In contrast to other host cells, CD8+ Cytotoxic T Lymphocytes (CTLs) eliminate virally-loaded cells from host by secreting a range of cytokines including interferons (IFNs), granzyme, and perforin. Overall removal of viral particles is mediated by CD4+ helper T cells (Th), cytotoxic T cells, and B cells [15]. As mentioned, patients with SARS-COV-2 were found to have a significantly reduced lymphocyte pool in peripheral blood than healthy individuals. Patients with long-term SARS-COV-2 infections presented with cardiac and renal symptoms showed a substantial increase in CD8+ population compared to control. Active SARS-COV-2 infection had significantly raised CD19+ population. Usual CD4:CD8 ratio (2:1) in health is expected to increase in active SARS-CoV-2 as CD4 population increases as a pre-requisite of humoral response. Interestingly, in the longer version of post-COVID-19, CD8+ population showed a remarkable increase, particularly in two cases of coronary – cardiac and renal presentations.
As discussed, an enhanced CD4:CD8 ratio is necessary for immune response of COVID-19-infected patients. As observed in the study, an increase in CD8+ levels led to an altered CD4:CD8 ratio, a possible marker of longer version of the viral infection T cells increase cytotoxic activity by increasing the CD8+ population [16]. Observation showed a clear predominance of an unexplained surge in CD8+ expression, without a parallel increase in the CD4+ population in long post-SARS-CoV-2 patient showing cardiac presentation and possibly may indicate an unexplained non- obvious residence of one or more viral components in undefined host cells. This may as well be entirely unrelated to the two-year-old COVID incidence while at the same time it may also be a long unreported sequel of SARS-COV-2 infection, where structural or non-structural protein(s) may remain immunologically hidden in any or more of the host cell populations. The question stands.
While providing the major share of possibility of the unexplained issue as to whether any or more substrains or parts thereof may remain in molecular hibernation (as in HIV) for much longer periods than the presently prescribed post-COVID-19 time window of 3-6 months , to a logical uncertainty and further scientific scrutiny, it is only rational to be on guard both from diagnostic and from therapeutic directions with continued search for any left- behind structural or non-structural proteins which can justify such sudden sectarian surge of cytotoxic CD8+ cells which are primarily meant for cytotoxic elimination of virus loaded host cells.
Also questions remain is it that humoral response is a transient phenomenon operational only during active phase of infection or for a short period thereafter , and long term events are all CMI response with skewed CD4:CD8 ratio ? Is it that Ab titer is again a transient phenomenon with rapid decay in titer, specificity and affinity? To answer these questions IgG characterization–both quantitative as well as mapping of the hyper variable domains is the next phase of work.
Long post-SARS-COV-2 cases are difficult in identification because of absence of any universal clinical marker(s). In addition to CD marker(s) this study has probed functional cellular integrity through assessment of energy metabolism. Mitochondrial wall integrity was assessed through JC-1 dye accumulation status (Figure 1). J-aggregates in control cells showed significant quantitative fluorescence difference versus patient sample thereby indicating loss of mitochondrial membrane integrity.
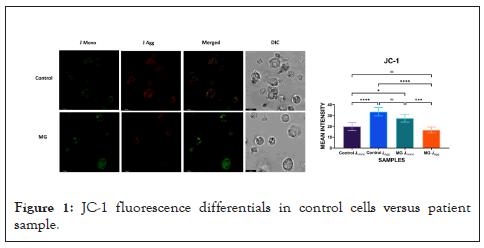
Figure 1: JC-1 fluorescence differentials in control cells versus patient sample.
Long SARS-COV-2 is an elusive clinical entity thus far in time of appearance, types of organs affected, extent of clinical presentations, nature of ending and in molecular leave-behinds if any. Also there is a need of a reasonably identifiable marker capable of addressing non-specific organ affections across the board. This brief work attempts to draw attention to: the validity of currently accepted time window of 4-6 months post-primary infection to be called long COVID, only the CD8+ subset and its unexplained expansion without parallel increase in CD4+ population as a possible cellular marker Loss or impairment of mitochondrial membrane integrity, if any as shown as a representative data, could be a possible cause of impairment of physiological ROS production leading to repeat affections of invasive pathogens–both viral and microbial – post-primary SARS-COV-2 event. This being an initial clinical presentation , the possibilities mentioned are all subject to subsequent evaluation through large scale studies irrespective of any classical primary presentation because not all SARS-COV-2 events were copybook.
We would like to acknowledge NIPER Kolkata and Apollo multi- specialty hospitals providing the facility for the experimental work.
Ethical clearance was done by the ethical committee at Apollo multi-specialty hospital, Kolkata, following the ICMR guidelines.
There is no conflict of interest.
Funding is provided by NIPER Kolkata
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Datta D, Singh R, Ghosh D, Chakravarti R, Somasundaram A, Dutta P, Ravichandiran V, Ray U, et al. (2022) SARS-COV-2: Long COVID Redefined in Time and Marker?. J Clin Cell Immunol.13:671.
Received: 13-Sep-2022, Manuscript No. JCCI-22-19181; Editor assigned: 16-Sep-2022, Pre QC No. JCCI-22-19181 (PQ); Reviewed: 03-Oct-2022, QC No. JCCI-22-19181; Revised: 10-Oct-2022, Manuscript No. JCCI-22-19181 (R); Published: 17-Oct-2022 , DOI: 10.35248/2155-9899.22.13.671
Copyright: © 2022 Datta D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.