
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2023)Volume 6, Issue 2
Traceless synthesis of 2-aminoimidazoquinoxalinones has been performed on soluble polymer support under openvessel microwave dielectric heating. The reaction progression is monitored directly by the conventional proton Nuclear Magnetic Resonance (NMR) without release of the substrate from the support. Fmoc-deprotected amino acid polymer conjugates react with 1,5-difluoro-2,4-dinitro benzene to yield polymer bound dinitro fluoro amines, which are further substituted by various primary amines to yield PEG-immobilized dinitro diamines. Simultaneous reduction of aromatic meta-dinitro group leads to the traceless release of 2-qunoxalinones, followed by N-hetero cyclization with various isothiocyanates in the presence of mercury (II) chloride to furnish 2-aminoimidazoquinolinone rings with three points of diversity at rapid pace.
Traceless liquid-phase strategy; Tricyclic quinoxalinone imidazole; Open-vessel microwave irradiation; Polyethylene glycol
Heterocyclic derivatives with polycyclic skeletons such as tricyclic quinoxalinones play an important role in the arsenal of clinically useful therapeutic agents (Figure 1) [1-3]. This 2-aminoimidazoquinoxalin constitute an interesting chemical space of drug-like derivatives since a small variation of the structure can lead to a profound change in the receptor binding profile or clinical activity [4]. Imidazo [4,5-g] quinoxalin comprises a imidazoquinoxaline scaffold with variable moiety at the second position to inhibit the activities of Akt/PKB isoforms selectively. Diamnobenzobisthiazoles containing bis-tertiary amino side chain show good in vivo inhibition against the swelling of the uninjected paw in the prophylactic adjuvant arthritis model orally [5-7]. Imidazol [4,5-g]quinoxaline have been identified as the potential inhibitors against testis-specific serine/threonine kinase1. Design concept of the present library has originated from the observation of the extensive biological activities of the quinoxalin-2(1H)-one and 1H-benzo[d]-imidazol-2-amine moiety which are attractive privileged scaffolds. The creation of a hybrid quinoxaline-related tricycle scaffold composed of quinoxalinone and 1H-benzo[d]-imidazol-2- amine thus, has a substantial intellectual appeal resembling druglike molecules. Therefore, combination of these two privileged pharmacophores may provide additional opportunities to discover new lead compounds.
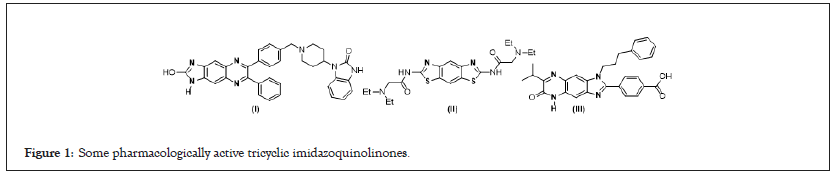
Figure 1: Some pharmacologically active tricyclic imidazoquinolinones.
Drug discovery is a time-consuming and expensive process. The bottleneck lies in the generation of compound libraries with diversity of various chemical spaces. Substituted heterocyclic compounds offer a high degree of structural diversity and have proven to be broadly useful as therapeutic agents. As a result, an increasing range and number of pharmaceutically interesting heterocyclic compounds have been prepared rapidly using combinatorial chemistry to keep pace of high throughput biological screening [8]. Application of solid phase combinatorial synthesis for the rapid generation of small molecules continues to be areas of great interest [9].
In providing a viable alternative to the solid support reaction, liquid phase synthesis has been signifying the usefulness of employing a soluble macromolecular support as a carrier such as Polyethylene Glycol (PEG). Polyethylene glycol is inexpensive than that of other soluble polymer supports such as polyethylene oxide and polyoxyethylene. Soluble polymer supported synthesis of small molecules benefits the advantages of both solution phase and solid phase syntheses. Various synthetic steps are conducted under homogeneous conditions with favorable reaction kinetics, whereas purifications are performed just by filtration and polymer precipitation. This alternative strategy, known as liquid-phase combinatorial synthesis, has been investigated to synthesize various heterocyclic combinatorial libraries [10,11]. Soluble polymer supported molecules are directly amenable to standard spectroscopic methods such as nuclear magnetic resonance spectroscopy and mass spectrometry which allows to perform in situ reaction monitoring without the need to cleave the compound from the polymeric support [12].
Although liquid phase organic synthesis can decrease the time of library generation by simplifying the purification procedure. The optimization of reaction condition is still a time-consuming process. During last two decades, microwave assisted organic synthesis have been demonstrated in drastically accelerating a variety of synthetic transformations. Microwave assisted chemical synthesis attributes to the application of electromagnetic radiation within the microwave frequencies to convey the energy to start, force or promote some chemical reactions. The main benefits of performing reactions under microwave irradiation are the significant rate enhancement and the higher product yields that can frequently be observed [13]. Given the current demand for novel compounds in drug discovery, the combination of microwave assisted organic synthesis and traceless liquid phase synthetic strategy improve the efficiency of library generation importantly.
In this report, we described a scaffold-directed approach to prepare benzofused chemical libraries using 1,5-difluoro-2,4-dinitrobenzene as a multifunctional building block [14,15]. To extend the scope of our recent studies, we report herein an efficient cyclization- cleavage method to simplify the synthetic strategy as well as to reduce the synthetic steps. After liberating from the support, the desired products are easily recovered, ignoring the requirement of post cleavage workup. Hence heterocyclic compounds of interest can be generated in free of any trace of the linker used to tether the starting building block to the polymer support. In view of our ongoing program for the development of novel synthetic strategy toward heterocyclic compounds, we herein report the microwave assisted traceless synthesis of tricyclic quinoxalinone imidazoles libraries with three points of structural diversity.
Poly ethylene glycol 1 (MW ~ 5000, 200 μmoles of free OH groups/g) is used as the soluble polymer support for the microwave assisted synthesis as a test of imidazoquinoxalinones. Using the stoichiometry test of reagents, the coupling reaction was complete in 8 hours by conventional reflux heating. The emergence of the free amino group was induced by treating with 10% piperidine in dichloromethane at room temperature which left the polymer support intact and led to the polymer-bound amines. During NMR monitoring reaction progress, the signals of the two methyl groups of the L-valine were nonequivalent. One of the two fluorines of the 1,5-difluoro-2,4-dinitrobenzene was substituted by the nucleophilic polymer-bound aminoester in the presence of triethylamine. This ipso-fluoro displacement reaction was achieved in 7 minutes under microwave irradiation condition. It should be mentioned that no di-substituted species were observed under harsh microwave dielectric heating. The evidence is based on the integration ratio of Ha and Hb which shows 6:1 instead of 12:1. The immobilized polymer conjugate may be too bulky to precede second ipso-fluoro displacement with polymer-bound aminoester. Furthermore, compound became less reactive than difluoronitrobenzene once one fluorine atom is replaced by polymer bound amino esters (Figures 2 and 3).
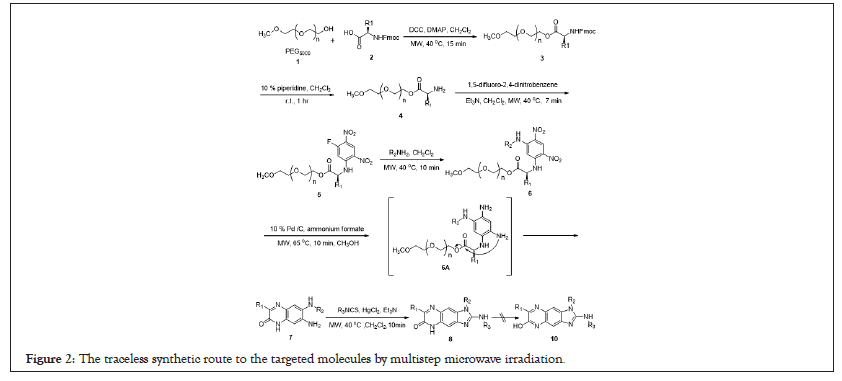
Figure 2: The traceless synthetic route to the targeted molecules by multistep microwave irradiation.
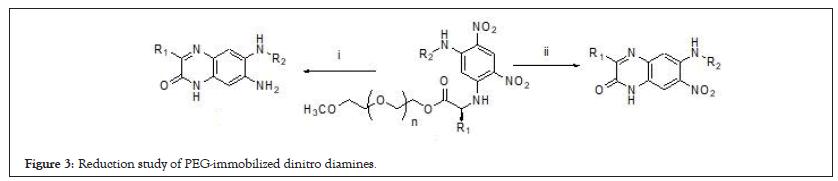
Figure 3: Reduction study of PEG-immobilized dinitro diamines.
After Nucleophilic Aromatic Substitution reaction (SNAr), the new peaks of Hb, Hc and Hd in the aromatic region were emerged. Displacement of the remaining fluorine atom by various primary amines introduced the second point of diversity to give 6 in high yield. The reactions went smoothly under open vessel MW conditions (150W) for 10 minutes and no side reactions such as cleavage of the polymer support or the racemization of the chiral center were observed. Comparison of spectra B and C in, Hb (doublet, specrum B) is upfield shifted by 0.9 ppm to 5.55 ppm (singlet, spectrum C) which implied the weak electron donating fluoro atom was transformed into strong electron donating amine functionality.
To completely reduce the aromatic m-dinitro group of PEG-immobilized dinitro diamines to the corresponding o-phenylenediamine derivative, various reduction conditions were investigated systemically including H2-Pd/C, SnCl2.7H2O and Zn/ HCOONH4 [16-18]. All the above-mentioned methods delivered directly 2-quinoxalinone analogues, an o-nitroaniline intermediate without further reduction of another nitro group (Figure 3). It is also not possible to convert intermediate to aniline using the same reduction conditions. This is implied that the resulting polymer bound triaminobenzene was cyclized spontaneously after reduction and aromatized to give the compound. It cannot be further reduced to di-amino compound either by refluxing or microwave irradiation. These results may indicate the difficulty to reduce di-nitro groups because of double resonance effect of the electron donating amino groups in the ortho and para positions. However, simultaneously reduction of m-dinitro functionalities and traceless cleavage of 7-amino-3-alkyl-6-(alkyllamino) quinoxalin- 2(1H)-one from the support was finally achieved with palladium black in the presence of ammonium formate in methanol [19]. The spectroscopic evidences were the disappearance of the signals of polymer support and Hc was upfield shifted to 7.08 ppm from 9.2 ppm. Crude 3,7-dialkyl-6-(alkyllamino)quinoxalin-2(1H)- one directly released from the support were cyclized with various isothiocyanates in the presence of HgCl2 under the open-vessel microwave heating (200 W) for 10 minutes. However, it took 16 hours for the construction of benzoimidazol-2-amine moiety under conventional refluxing condition. The mechanism toward the formation of benzoimidazol-2-amines was deduced that the sulfur atom of isothiocyanates was chelatd to mercury ion to generate highly electrophilic species in situ. The nucleophilic diamines then attacked on the electrophilic species and extruded H2S to afford the imidazoquinoxalinones. By the use of Thin Layer Chromatography (TLC), we observe that the less lipophilic diamines were gradually transformed into more lipophilic imidazoquinoxalinones. The 1H NMR monitoring model also showed the downfield shift of Hb as well as Hc and the newly appearance of phenyl group in the 7 ~ 8 ppm region. Quinoxalin-2(1H)-one and quinoxalin-2-ol were the possible proposed skeletons for the final target compound. The IR spectrum showed an amide absorption in the range of 1690-1640 cm-1 which indicates that it was a 3,4-dihydroquinoxalin-2-one but not a quinoxalin-2-ol. This multi-step microwave-assisted synthesis and traceless strategy have offered an efficient and convenient approach toward the access the tricyclic imidazoquinoxalinones analogues. Seventeen examples with three different appendages were synthesized in good yields and all the experimental data comprising MS, 1H NMR, and 13C NMR were coincident with these products.
In summary, we have demonstrated a high throughput platform for the rapid generation of imidazoquinoxalinone libraries with three points of diversity by the synergistic application of multistep microwave irradiation and traceless liquid phase synthesis. The libraries of compounds are usually obtained in high purity and yield just by washings of each polymer attached intermediates and simple precipitation with minimum column purification. The reaction progress of forwarding synthetic route on the soluble support is successfully monitored by conventional proton NMR spectroscopy without the and cleave and analyze method. The coupling of microwave technology with a liquid-phase traceless synthetic strategy constitutes a novel and efficient approach for the rapid generation of pharmaceutical interesting small molecules. The tricyclic 2-aminoimidazoquinoxalinone library is ready for in vitro biological screening and the results will be reported in due course.
The authors thank the National Science Council of Taiwan for financial assistance, and the authorities of the National Chiao Tung University for providing the laboratory facilities.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Thummanagoti S (2023) Scaffold-Directed and Traceless Synthesis of Tricyclic Quinoxalinone Imidazoles under Microwave Irradiation. J Clin Chem Lab Med. 6:267.
Received: 15-May-2023, Manuscript No. JCCLM-23-22173; Editor assigned: 17-May-2023, Pre QC No. JCCLM-23-22173 (PQ); Reviewed: 31-May-2023, QC No. JCCLM-23-22173; Revised: 07-Jun-2023, Manuscript No. JCCLM-23-22173 (R); Published: 14-Jun-2023 , DOI: 10.35248/JCCLM.23.6.267
Copyright: © 2023 Thummanagoti S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.