Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Editorial - (2016) Volume 5, Issue 1
Natural products are not necessarily gentle medicines. A famous example is the so-called “Chinese nephropathy”. In 1993, nephrotoxicity of more than 100 women under the age of 50 was observed in Belgium as a consequence of herbal poisoning. The use of Chinese herbs containing aristolochia acid in a slimming regime resulted in extensive interstitial fibrosis of the kidney, which led to renal failure and in some cases to renal urotheliomas [1,2]. Although not all potentially toxic substances cause such strong side effects, there is still uncertainty about herbal products and reports about contaminations and adverse side effects might sometimes leave a bad after taste about the use of herbal products [3,4].
Therefore, strict quality controls of natural products including preclinical and clinical evidence of safety and efficacy are of major importance [5]. For that reason, the European Parliament and Council established the traditional herbal medicinal products directive (THMPD), which was recently adjusted [6]. Since April 2011, traditional herbal medicinal products have to undergo the Good Manufacturing Practice (GMP) and should no longer be categorized as food or foods supplements, and therefore underlie strict regulations to guarantee safety and to give confidence in natural products back to the public.
However, the implementation of quality control for nutritional supplements and herbal over-the-counter products is difficult, and the nescience about potential risks can lead to toxication by selfmedication [4]. Recently, public media reported on the discovery of pyrollizidine alkaloids (PA) in honey and medicinal herbs, which can be metabolized to electrophilic pyrroles and lead to liver and lung damage [7,8]. As a result, the consumption of certain honey products was announced to be toxic.
Another example of herbal poisoning related to contaminated traces in honey dates back to the year 401 B.C. The ancient Greeks observed that soldiers suffered from honey and were unable to move on [9]. This unexpected effect of contaminated honey was utilized years later as a tactical strategy by the Turk [10] and is based on the presence of grayanotoxins in the so called “mad honey”.
Grayanotoxins are natural products mostly found in plants of the Ericaceae family, especially in the Rhododendron, Pieris, Agarista and Kalmia genera [9-11]. Mad honey poisoning is usually associated with the Eastern Black Sea region of Turkey, but was described in the nineteenth century in Europe and North America as well. Grayanotoxins are polyhydroxylated cyclic diterpenes with more than 25 isoforms known, isolated from Rhododendron.
The primary toxic effect of grayanotoxins is due to their binding capability to voltage-gated sodium channels, leaving them in an activated state followed by hyperpolarization [10]. Therefore, ingestion of honey containing grayanotoxins results in hypotension, bradycardia, nausea or vomiting, dizziness, impaired consciousness and can be treated in severe cases with atropine as an antidote to prevent mortality. The symptoms usually remain for 1-2 days.
In contrast to drug development in pharmacology, where ligands should be as selective as possible, the evolutionary aim of the plant is to defeat itself against a broad spectrum of predators and other enemies. This is the main reason, why natural products are rather multi- than monospecific [12]. Therefore, it is not astonishing that novel targets can also be identified for grayanotoxins. As shown by in silico molecular docking analyses, grayanotoxins are able to bind to tubulin (Table 1).
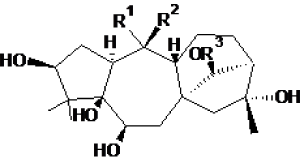 |
Lowest binding energy (kcal/mol) | Pki-value (µM) | Amino acids involved in H-bonds | Amino acids involved in hydrophobic interactions | ||||
|---|---|---|---|---|---|---|---|---|
| Test compounds | R1 | R2 | R3 | Chain | ||||
| Grayanotoxin 1 | OH | CH3 | Ac | α-Tub | -8.04 ± 0.03 | 1.28 ± 0.05 | ASN258 | THR257, VAL260, MET313, CYS315, TRP346, CYS347, PRO348, GLY350, PHE351, LYS352, |
| β-Tub | -7.37 ± 0.01 | 3.97 ± 0.04 | SER230, LEU273 | LEU215, HIS227, ALA231, SER234, PRO272, ARG318, PRO358, LEU361 | ||||
| Grayanotoxin 2 | CH2 | H | α-Tub | -7.47 ± 0.04 | 3.4 ± 0.2 | PRO261, TRP346 | THR257, ASN258, VAL260, TYR262, MET313, ALA314, CYS347, PRO348 | |
| β-Tub | -7.153 ± 0.006 | 5.72 ± 0.04 | PRO272, ARG359, LEU361 |
HIS227, ALA231, LEU273, PRO358, GLY360 | ||||
| Grayanotoxin 3 | OH | CH3 | H | α-Tub | -7.57 ± 0.03 | 2.8 ± 0.1 | GLU254, MET313, CYS315, PHE351 |
ASN258, ALA314, CYS347, PRO348, THR349, GLY350, LYS352 |
| β-Tub | -7.133 ± 0.006 | 5.91 ± 0.03 | LEU228, SER230, ARG359 |
HIS227, ALA231, PHE270, PRO272, LEU361 | ||||
| Grayanotoxin 4 | CH2 | Ac | α-Tub | -8.11 ± 0.08 | 1.1 ± 0.2 | CYS315 | ASN258, VAL260, PRO261, ALA314, TRP346, PRO348, THR349, GLY350, PHE351, LYS352 | |
| β-Tub | -8.91 ± 0.06 | 0.29 ± 0.03 | HIS227, SER234, LEU273, ARG359 |
VAL23, LEU215, LEU228, SER230, ALA231, PRO272, ARG318, PRO358, LEU361 | ||||
| Control drugs | Chain | |||||||
| Colchicine | β-Tubulin | -7.44 ± 0.01 | 3.50 ± 0.06 | / | ASN102, ARG105, ASP197, VAL258, PRO261, LEU263, HIS264, HIS406, TRP407, GLY410 | |||
| Paclitaxel | β-Tubulin | -9.8 ± 0.6 | 0.09 ± 0.07 | ARG276 | LEU215, LEU217, ASP224, HIS227, LEU228, ALA231, PRO272, LEU237, THR274, GLN279, TYR281, LEU284, GLY360, LEU361 | |||
| Vinblastine | β-Tubulin | -8.94 ± 0.07 | 0.28 ± 0.03 | ARG276, ARG359 | GLU22, VAL23, SER25, PHE81, HIS227, ALA231, PRO272 |
|||
Table 1: Docking results of grayanotoxins I-IV, colchicine, paclitaxel and vinblastine to tubulin. Shown are the lowest binding energies, the Pkivalue and the amino acids involved in H-bonds and hydrophobic interactions. The docking was performed as previously described [18].
Tubulins consist of two subunits, α- and β-tubulin, and polymerize to microtubules as an essential constituent of the cellular cytoskeleton and mitotic machinery [13]. Known inhibitors of microtubules are colchicine [14], paclitaxel [15] and vinblastine [16], which bind to the β-tubulin subunit (Figure 1) and lead to apoptosis. Paclitaxel and vinblastine are therefore used as anticancer drugs. Due to its intolerable high toxicity, colchicine is not suited for cancer therapy, but can be used at low doses to inhibit monocytes in gout and rheumatic diseases.
At this point, the question arises, whether the toxic and otherwise therapeutically not suitable grayanotoxins might also be useful for cancer therapy (at high doses) or for gout (in low doses). It may sound provocative to ask the question, whether or not there is a possibility for a “second career” of grayanotoxins in the treatment of these diseases. However, molecular docking revealed similarly strong binding energies to tubulin for grayanotoxins compared to the known tubulin inhibitors. Especially for vinblastine- and paclitaxel-resistant cancers, grayanotoxins may become an alternative because of their preferred binding to the α-tubulin subunit, while the other two drugs bind to β- tubulin. At least for grayanotoxins I to III, the results clearly indicate a preferred binding to α-tubulin. Frequently, these three grayanotoxins, induce toxications such as the mad honey disease [11]. Thus, their electronic characteristics seem to differ from grayanotoxin IV, which shows better binding to β-tubulin. Further, in vitro investigations have to confirm this observation.
Additionally, grayanotoxins might be suitable candidates for nanotechnological approaches in cancer therapy [17]. Concerning their high toxicity, grayanotoxins could be specifically targeted to cancer cells, e.g. in the form of liposomes coupled with cancer cellspecific antibodies at reduced adverse side effects in normal tissues.
In conclusion, toxic compounds such as grayanotoxins may still reveal the therapeutic potential for targeted chemotherapy of cancer.