
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2023)Volume 14, Issue 2
Background: Mucosal immunity plays a major role not only in the prevention but probably also in the outcome of COVID-19. An enhanced production of secretory Immunoglobulin A (sIgA) might contribute to the activation of the immune response mechanisms.
Objective: To assess the levels of sIgA produced by epithelial cells in the nasal and pharyngeal mucosa and those measured in salivary gland secretions and to study the course of COVID-19 following the intranasal or subcutaneous administration of a bacteria-based immuno-stimulant agent.
Materials and methods: This study included 69 patients aged between 18 and 60, who had moderate COVID-19 infection. They were divided into two groups: Group 1 (control group) included 39 patients who received only background therapy, and Group 2 was made up of 30 patients who received background therapy in combination with the Immunovac VP4 vaccine, a bacteria-based immuno-stimulant agent, which was given for 11 days starting from the day of admission to hospital. The levels of sIgA were measured by ELISA in nasal epithelial swabs, pharyngeal swabs and salivary gland secretions at baseline and on days 14 and 30.
Results: The convalescence phase of moderate COVID-19 was associated with a decrease in sIgA levels in nasal swabs, persistently high sIgA levels in salivary gland secretions and no changes in pharyngeal swabs with the levels similar to those in healthy subjects. The addition of an immuno-stimulant agent to combination therapy for patients with COVID-19 stimulates the production of sIgA in the nasal and pharyngeal compartments, reduces C -reactive protein (CRP) levels and shortens the duration of fever and the length of hospital stay.
Conclusion: Using an immuno-modulatory agent containing bacterial ligands in therapy for COVID-19 patients enhances the production of sIgA in the nasal and pharyngeal compartments and improves the course of the disease.
COVID-19; Mucosal immunity; sIgA; Microbial-based immuno-modulatory agent; Immune therapy in COVID-19
The mucosal immunity plays a critical role in preventing droplet infections, including SARS-CoV-2. In case of SARS-CoV-2, infection is, however, facilitated by some structural features of the virus and the fact that it engages Angiotensin-Converting Enzyme 2 (ACE2) as the primary receptor and employs the Trans-Membrane Serine Protease 2 (TMPRSS2) for protein priming [1]. The induction of mucosal immunity is in the future likely not only to become a strategy in preventive vaccination against particular infections.
e.g. SARS-CoV-2 infection, but also a treatment strategy, i.e. a tool for restoration of a balanced profile of immuno-competent cells, which are responsible for limiting the spread of infection and localizing it at the site of entry at earlier stages. Despite ongoing research of the mechanisms of mucosal immunity in viral infections, in particular coronavirus infection, the approaches to immune therapy and the role of immuno-biological medications in the activation of mucosal immunity during the active inflammation stage have not been fully investigated.
Previous clinical studies showed that most microbial-based immuno-modulatory agents have a highly favorable safety profile and are effective in reducing the signs of active respiratory infections. In some cases these agents can reduce the need for antibiotics and other medications while maintaining the treatment performance [2-6]. It is believed that the recognition of bacterial antigens included in such formulations by dendritic cells activates immune response and stimulates the production of antibodies by B-cells, which is accompanied by the enhancement of phagocytic activity of macrophages and polymorphonuclear neutrophils as well as an increased production of lysozyme and secretory immunoglobulin A (sIgA) [7,8]. Administration of bacteria-based immuno-modulatory agents induces polarization of immune response, mainly type Th1, and is associated with an increase in NK cytotoxicity and an enhanced expression of TLR2, TLR4 and TLR9 [9]. So far, a large number of papers have been published focusing on the use of bacteria-based vaccines in the treatment of obstructive pulmonary disease and asthma [10-13]. The results of the studies investigating the efficacy of immuno-modulatory agents in COVID-19 infection are, however, scarce, anecdotal, and sometimes not evidence-based. It has been supposed that products containing bacterial ligands used as part of a combination treatment help maintain high levels of sIgA throughout the treatment period [14]. Elevated levels of nasal sIgA may activate the mechanisms of mucosal immune response and contribute to a favorable course and outcome of COVID-19 disease [15]; there may also be a correlation between sIgA levels and the clinical symptoms. Cervia et al. [16] showed that systemic antibody production against SARS-CoV-2 develops mainly in patients with severe COVID-19, with very high IgA titers seen in patients with severe acute respiratory distress syndrome, whereas mild disease may be associated with transient production of SARS-CoV-2–specific antibodies but may stimulate mucosal SARS-CoV-2–specific IgA secretion. In other words, IgA-mediated mucosal immunity may be a critical defense mechanism against SARS-CoV-2 at the individual level that may reduce the virus infectivity and prevent its shedding [17].
Therefore, development of new ways to induce the production of post-infection antibodies by influencing the mucosal components of the innate and adaptive immune system of the respiratory tract in patients with novel coronavirus infection can be relevant for treatment decision-making in COVID-19.
Objective of the paper
To assess the levels of sIgA produced by epithelial cells in the nasal and nasopharyngeal mucosa and those measured in salivary gland secretions and to study the course of COVID-19 following the intranasal or subcutaneous administration of a bacteria-based immuno-stimulant agent.
Clinical study design
The primary objectives were to evaluate the changes in sIgA levels in different compartments of the upper respiratory tract in COVID-19 patients over the period between admission to hospital and discharge (from day 1 to day 14) as well as to study the effects of a bacteria-based immuno-stimulant on the secretion of sIgA, the duration of fever, the number of hospital days, and the CRP level. The secondary objective included analysis of sIgA levels in patients with COVID-19-asociated lung disease 30 days after the start of treatment, depending on the treatment regimen.
A total of 69 patients were included in the study. They were divided into the following groups: Group 1 (n=39) included patients who received only background therapy and Group 2 (n=30) was made up of patients who received background therapy in combination with Immunovac VP4 vaccine, a bacteria- based agent.
This was a Phase IV controlled non-randomized post-marketing study. It was conducted in a dedicated COVID-19 hospital in Moscow (Russian Federation). Patients were selected after medical tests, physical examination and an assessment of the inclusion and exclusion criteria as well as the indications and contraindications for Immunovac VP4 as per the package insert. Selection of patients was also performed in accordance with the information provided in the formal Provisional Guidelines “Prevention, diagnosis and treatment of novel coronavirus infection (COVID-19)” approved in the Russian Federation. Patients were followed up for a minimum of 30 days. All treatment information, physical examination findings and test results were reported using standard medical records (individual patient documentation).
Legal and ethical conduct of study
Treatment was carried out in accordance with the Provisional Clinical Guidelines "Prevention, diagnosis and treatment of novel coronavirus infection (COVID-19)" developed by the Ministry of Health of the Russian Federation and clause 20 "Voluntary Informed Consent to Medical Intervention and Refusal of Medical Intervention" (Federal Law No. 323-ФЗ, dated November 1, 2011 "On Fundamental Healthcare Principles in the Russian Federation" (as amended on April 3, 2017).
The study protocol was approved on November 26, 2020 by the local Ethics Committee of the Federal State Budgetary Scientific Institution I.I. Mechnikov Research Institute of Vaccines and Sera (Russian Federation). The study was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonization's Good Clinical Practice guideline and Russian regulatory requirements. Written informed consent was obtained from patients prior to their enrollment in the study.
Patients
A total of 69 inpatients, aged between 18 and 60, who had confirmed COVID-19 infection with moderate lung involvement were included in the study. SARS-CoV-2 infection was confirmed by PCR of nasopharyngeal swabs and/or clinical and X-ray findings (all patients had Computed Tomography [CT] signs of lung injury such as ground-glass opacities and areas of consolidation consistent with grade 2 CT scan [25%-50% lung involvement]). The COVID-19 patients included in the study met all the inclusion criteria and did not meet the exclusion criteria. They received background therapy which was selected according to the severity of their disease and as recommended by the clinical guidelines developed by the Ministry of Health of the Russian Federation. It included Favipiravir 200 mg (standard regimen), enoxaparin 0.4 mg/day, subcutaneously, dexamethasone 8-12 mg/day and tocilizumab 400 mg/day (for patients with CRP ≥ 60 mg/L).
The patients were randomly assigned to two groups. Group 1 (control group) consisted of 39 patients (26 males and 13 females), the median age was 42 (33–54) years. These patients received only basic background therapy.
Group 2 was comprised of 30 patients (22 males and 8 females), the median age was 42 (37–45) years. These patients received Immunovac VP4 vaccine, a bacteria-based immuno-stimulant, as an add on to the background therapy. This vaccine was given starting on day 1 of hospitalization after careful consideration of all indications and contraindications as per the package insert.
These groups of patients with moderate pneumonia were matched by age (42 (33–54) years in the control group and 42 (37–45) years in the Immunovac VP4 group, p=0.79), gender (26/13 male/female in the control group and 22/8 male/female in the Immunovac VP4 group, p=0.33), and the number of days after the onset of disease (5 (4–8) days in the control group and 5 (3–7) in the Immunovac VP4 group, p=0.63). They were also matched by body mass index, amount of impaired lung parenchyma, and laboratory findings.
Samples were also taken from different compartments of the upper respiratory tract of healthy unvaccinated healthcare workers who had not been exposed to SARS-CoV-2 (n=10). The study parameters were measured in these samples; median values were calculated and considered as median reference values.
Inclusion criteria: Inpatients aged between 18 and 60 with confirmed COVID-19 infection, i.e. SARS-CoV-2 detected in a nasopharyngeal swab by PCR and/or clinical and X-ray confirmation (all patients had CT signs of lung injury such as ground-glass opacities and areas of consolidation consistent with grade 2 CT scan (25%-50% lung involvement)), and signed and dated informed consent.
Exclusion criteria: Patients were excluded if they met any of the following criteria: lung abscess, pleural empyema, active tuberculosis; severe birth defects or serious chronic disorders, including exacerbations/decompensation of chronic disorders, such as pulmonary, liver, renal, cardiovascular, neurological, or mental disorders, malignancies within the last five years, metabolic diseases; HIV or hepatitis B or C; use of immunoglobulin or blood transfusion within the last three months prior to the start of the study; long use (more than 14 days) of immuno-suppressive or other immuno-modulatory drugs within six months prior to the start of the study; any known or suspected immunosuppressive or immunodeficiency disorder or active autoimmune disease; any vaccination within the last month; acute respiratory infections less than one month prior to study; pregnancy or lactation; simultaneous participation in another clinical study; or the patient’s inability to comply with the study protocol requirements (as judged by the investigator).
Study drug
Immunovac VP4 vaccine: This is a polyvalent vaccine based on the antigens of opportunistic microorganisms (mixture of water soluble antigens extracted from Staphylococcus aureus, Klebsiella pneumoniae, Proteus vulgaris and Escherichia coli). This product is approved for subcutaneous use (Registration Certificate # ЛСР-001294/10 issued by the Ministry of Health of the Russian Federation on February 24, 2010) as well as nasal and oral use (Registration Certificate # ЛСР-001293/10 issued by the Ministry of Health of the Russian Federation on February 24, 2010). It is manufactured by Scientific and Production Association for Immunological Preparations “Microgen”, a federal state unitary enterprise (Ufa, Russian Federation).
Pharmacological properties: It is a bacteria-based immuno-stimulant. Its mechanism of action is due to the activation of the key effectors of innate and adaptive immunity. This vaccine enhances phagocytic activity of macrophages, optimizes T-cell counts and functional activity of lymphocyte subsets (CD3+, CEM, CD8+, CD16+ and CD72+), programs CD4+ T-cells to proliferate and differentiate into Th1 cells, stimulates the production of IFN-γ and IFN-a and improves the production of immunoglobulin isotypes by inhibiting IgE synthesis and inducing IgG, IgA and sIgA synthesis. It induces the production of antibodies to four opportunistic microorganisms whose antigens are included in the composition. It also provides cross protection against Streptococcus pneumoniae, Haemophilus influenzae and other pathogens due to the existence of common antigen components. In terms of clinical outcomes, vaccination reduces the rate of acute infections, duration of infection, severity of symptoms, risk of exacerbation of chronic diseases and the amount of medication treatment.
This vaccine is administered using the following combined regimen: intranasal administration followed by oral administration. It can also be administered subcutaneously. Immediately prior to use, 2 mL of solvent (0.9% sodium chloride for injection or boiled water brought to 18-25°С) is added to the vial with a syringe, and the contents is mixed. The product is instilled into the nasal cavity using a medical dropper. For oral use, the required amount of vaccine is drawn from a vial with a syringe and then transferred into a spoon.
Drug-drug interactions: The product can be used with other medications as part of combination treatment. It can be administered in combination with antibiotics, antiviral, antifungal and antihistamine agents, bronchodilators, corticosteroids, and β-adrenoceptor agonists. Patients who receive immune therapy or immune-prophylaxis with Immunovac VP4 should not receive any other immuno-modulatory agents within one month prior to this course of therapeutic or preventive treatment and within three months after its completion.
Schedule, dose and timing for vaccination: When prepared, the solution of Immunovac VP4 was administered to patients at a dose of 2 drops (1 mg) in each nostril daily and subcutaneously every other day at doses 0.05 (0.5), 0.1 (1.0), 0.2 (2.0), 0.2 (2.0), 0.3 (3.0) and 0.3 (3.0) from day 1 to day 11 of hospital stay.
For all patients, demographic data, body mass index, symptoms of the disease, physical examination findings, results of laboratory tests (complete blood count, C-reactive protein, and blood coagulation profile) and other investigations (chest computed tomography), and concomitant diseases were assessed.
The severity of respiratory failure was defined by the blood oxygen saturation level measured by pulse oximetry (SpO2). Patients' nutritional status was assessed by body mass index, which was calculated using the standard formula: Body mass index=weight (kg)/height (m2). Pulse oximetry was performed using a pulse oximeter (series MD300C). Lung CT was performed on a spiral CT scanner Aquilion TSX-101A (Toshiba Medical Systems, slice thickness 1 mm, pitch 1.5) on admission and after 10 days of treatment.
Sampling
In study groups 1 and 2, samples were taken from different compartments of the upper respiratory tract: nasal mucosal epithelial scrapings, pharyngeal epithelial scrapings, and salivary gland secretions. Saliva was collected early in the morning before patients brushed their teeth and had a meal. Saliva was collected passively without any forceful coughing under supervision of a physician [18-20]. Sampling was performed in two steps: on study day 1 before study treatment was administered, on study day 14 and subsequently 30 days after the start of treatment.
Clinical laboratory tests, including CRP, were done in accordance with the institutional standards and patient’s condition.
Cytobrush sampling was performed in all patients to determine protein levels. Samples were collected using a type D brush (Yunona, Russian Federation) into three Eppendorf Tubes with sodium chloride solution. The tubes were centrifuged at 2000 g for about 5 minutes to sediment the epithelial cells and then refrigerated at +2-4°C until shipment to the laboratory, where the samples were examined within 24 hours of collection.
Levels of sIgA in all biological fluids were measured by enzyme-linked immuno-sorbent assay (Vector Best, Russian Federation). Plates were read using a Multiskan Ascent ELISA microplate photometer (Thermo Electron Corporation, Finland). Levels of immunoglobulins were measured by enzyme-linked immuno-sorbent assay based on a two-step sandwich enzyme immunoassay using Monoclonal Antibodies (mAb) against the secretory component linked to alpha chain of IgA. Standards with known concentrations of sIgA and the samples were added to the wells of a plate coated with an anti-sIgA mAb.
The plate was then incubated according to the test kit instructions. The intensity of developing color is proportional to the concentration of sIgA in the sample. The concentration of sIgA was calculated using the standard curve and the measured optical density values.
These tests were performed using certified equipment provided by the Research Equipment Sharing Center of the Federal State Budgetary Scientific Institution I.I. Mechnikov Research Institute of Vaccines and Sera.
Statistics
The normality of distribution of the quantitative variables was tested using the Shapiro Wilk's normality test. Most variables were found to have a non-normal distribution, therefore, descriptive statistics for quantitative variables included median and interquartile range, Me(Q1-Q3). The 95% confidence intervals were calculated for the differences between the medians at the two time points.
Changes over time in sIgA levels were compared between the study groups by using a linear mixed effects model, where group and time point were fixed factors, and patients were random factors. This model was created in the lme4 package [21]. When the model was created, goodness-of-fit tests (normality of distribution and homogeneity of variance in residuals) were conducted using the DHARMa package [22]. If these goodness-of-fit tests showed some problems, a Box-Cox transformation was applied to the initial dataset, then a corrected model was built and goodness-of-fit tests were run on the transformed data. These are modelling results for the pooled data obtained at three time points by applying type III ANOVA with Kenward-Roger approximation for degrees of freedom; these tests were performed using the lmerTest package [23]. All post-hoc tests were performed using corresponding contrasts in the calculated linear mixed-effects model with a Benjamini-Krieger-Yekutieli correction [24].
Individual quantitative variables were compared between the study groups using the Mann-Whitney test. The one-sample Wilcoxon test was used to compare the medians of quantitative parameters to the expected medians.
The level of statistically significant differences was defined as p ≤ 0.05. Calculations and graphics were carried out using GraphPad Prism (v.9.3.0, license GPS-1963924) and the statistical programming environment R (v.3.6, license GNU GPL2).
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Table 1 provides an analysis of changes in sIgA in the study COVID-19 patients over the period between admission to hospital and discharge and 30 days after the start of the study.
| Study group | sIgA at study baseline | Time points, µg/L - Me(Q1-Q3) | p values for changes over time5 | |
|---|---|---|---|---|
| After 14 days | After 30 days | |||
| Nasal swap (reference value 29.9 µg/L) | ||||
| Control1 | 91.3 (50.9-156.0) | 59.0 (21.9-146.5) | 30.2 (7.6-61.7) | p0-14= 0.13, p0-30= 0.02, p14-30= 0.002 |
| VP42 | 77.5 (37.0-91.5) | 46.7 (19.8-109.2) | 107.0 (44.9-164.5) | p0-14= 0.35, p0-30= 0.01, p14-30= 0.02 |
| p values for groups | p=0.08 | p=0.27 | p=0.002 | - |
| LMEM3 – Group: F=0.7, p(63.8)=0.42 Time: F=0.6, p(100.4)=0.56 Group × Time: F=10.8, p(100.4)=0.001 | ||||
| Pharyngeal swab (reference value 6.5 µg/L) | ||||
| Control | 6.6 (1.0-30.4) | 9.4 (1.1-25.3) | 7.9 (1.1-13.9) | p0-14=0.69, p0-30=0.29, p14-30=0.32 |
| VP4 | 1.0 (0.4-11.7) | 9.1 (0.9-17.9) | 19.6 (3.7-54.1) | p0-14=0.06, p0-30=0.09, p14-30=0.001 |
| p values for groups | p=0.11 | p=0.69 | p=0.05 | - |
| LMEM4 – Group: F=0.3, p(63.6)=0.62 Time: F=3.3, p(95.0)=0.04 Group × Time: F=6.2, p(95.0)=0.003 | ||||
| Salivary gland secretions (reference value 71.7 µg/L) | ||||
| Control | 156.8 (64.3-234.9) | 120.8 (54.3-172.9) | 150.9 (119.5-192.3) | - |
| VP4 | 177.3 (94.5-234.2) | 154.4 (87.8-184.1) | 148.4 (93.5-202.9) | - |
| LMEM5 – Group: F=0.2, p(59.8)=0.66 Time: F=0.8, p(95.2)=0.46 Group × Time: F=0.0, p(95.2)=0.97 | ||||
Note: 1Group of background therapy; 2Group of background therapy + Immunovac VP4; 3A Linear Mixed-Effects Model (LMEM) was used, where group and time point were fixed factors and patients were random factors. These are pooled results for three time points obtained by applying type III ANOVA with Kenward-Roger approximation for degrees of freedom; 4Calculations were done using pre-transformed data. Data transformation was performed using the Box[1]Cox method (λ=-0.12); 5Post-hoc tests (p values for changes over time were the values for comparison between time points and p values for groups were the values for comparison between the two study groups at each study point) were performed using corresponding contrasts in the calculated linear mixed-effects model with a Benjamini-Krieger-Yekutieli correction.
Table 1: Analysis of changes over time in sIgA levels in the study groups at the study time points.
Levels of sIgA in salivary gland secretions
The study groups did not show statistically significant difference in terms of either absolute salivary sIgA levels throughout the study period or their changes from baseline (Figure 1). Of note, in COVID-19 patients salivary sIgA levels were significantly higher than in healthy unvaccinated subjects over the entire study period (p<0.001 for comparisons between the values measured at each time point in each study group and the median reference value [71.7 µg/L]).
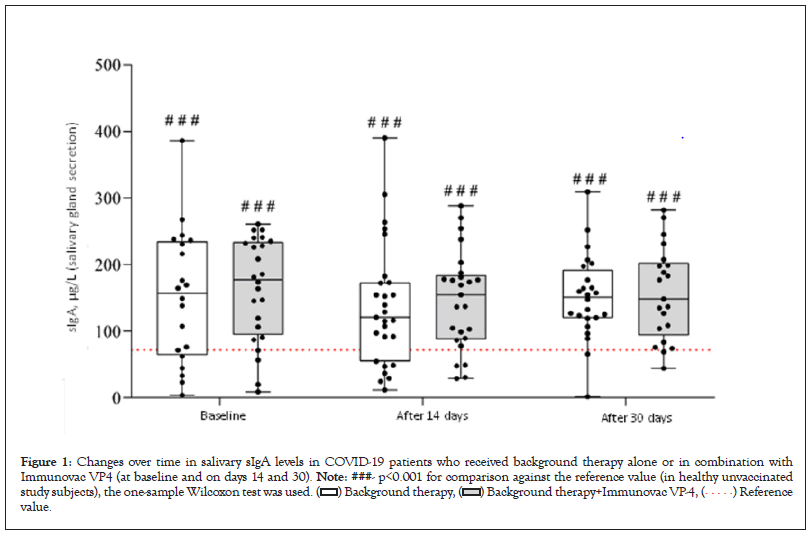
Figure 1: Changes over time in salivary sIgA levels in COVID-19 patients who received background therapy alone or in combination with
Immunovac VP4 (at baseline and on days 14 and 30). Note: ###- p<0.001 for comparison against the reference value (in healthy unvaccinated
study subjects), the one-sample Wilcoxon test was used.  value.
value.
Levels of sIgA in pharyngeal swabs
Evaluation of pharyngeal swabs revealed significant diverse changes in the sIgA levels for patients receiving and not receiving Immunovac VP4 (F=6.2, p(95.0)=0.003). In the control group, these levels did not show statistically significant difference from the baseline values throughout the study, whereas the patients who received Immunovac VP4 in addition to background therapy showed a significant increase from baseline in pharyngeal sIgA levels on day 30 after the start of the study (from 1.0 [0.4–11.7) µg/L to 19.6 (3.7–54.1) µg/L, p=0.001). Thirty days after the start of the study, pharyngeal sIgA increased by 18.1 (ranging from +0.8 to +27.1) µg/L in patients who received Immunovac VP4 in addition to background therapy compared to 1.3 [ranging from -6.1 to +3.3] µg/L in patients who received only background therapy; this difference was statistically significant (p=0.001). At baseline, the study groups did not show statistically significant difference in the pharyngeal sIgA levels (p=0.11). Of note, the baseline levels of pharyngeal sIgA in either study group did not significantly differ from those observed in the healthy unvaccinated subjects (p=0.25 and p=0.47 for comparisons of the values in the control group and the Immunovac VP4 group, respectively, to the median reference value [6.5 µg/L]). Nevertheless, 30 days after the start of treatment, patients in the Immunovac VP4 group had higher levels of pharyngeal sIgA than the patients who received only background therapy (19,6 [3.7–54.1] µg/L vs. 7.9 (1.1–13.9) µg/L, p=0.05) and healthy study subjects (p=0.01) (Figure 2).
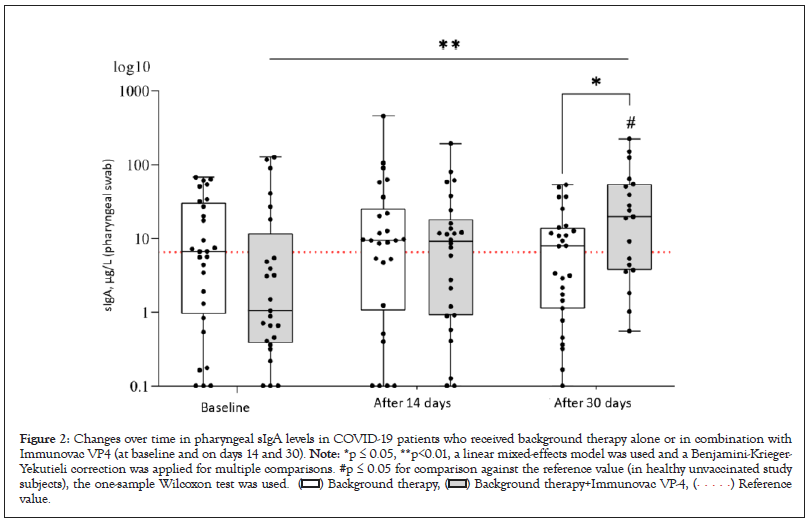
Figure 2: Changes over time in pharyngeal sIgA levels in COVID-19 patients who received background therapy alone or in combination with
Immunovac VP4 (at baseline and on days 14 and 30). Note: *p ≤ 0.05, **p<0.01, a linear mixed-effects model was used and a Benjamini-Krieger-
Yekutieli correction was applied for multiple comparisons. #p ≤ 0.05 for comparison against the reference value (in healthy unvaccinated study
subjects), the one-sample Wilcoxon test was used.  value.
value.
Levels of sIgA in nasal swabs
The most significant divergent changes in sIgA levels depending on the treatment administered were observed in nasal swabs (F=10.8, p[100,4]<0.001). At baseline, COVID-19 patients who received background therapy alone or in combination with Immunovac VP4 had similar sIgA levels in nasal swabs (p=0.08); however, 30 days after the start of the study patients who were given Immunovac VP4 in addition to background therapy had a statistically significant increase from baseline in this parameter (from 77.5 [37.0–91.5] µg/L to 107.0 [44.9–164.5] µg/L, p=0,02). In contrast, the control group showed a significant decrease in sIgA compared to the baseline values (from 91.3 [50.9–156.0] µg/L to 30.2 [7.6– 61.7] µg/L, p=0.002). Thus, on day 30 after the start of treatment, patients receiving Immunovac VP4 in combination with background therapy had statistically significantly higher levels of sIgA than patients who received only background therapy (p=0.002). On day 30 of the study, the change from baseline (the difference in medians) in sIgA levels was -61.0 (ranging from -84.3 to -28.6) µg/L in the control group and +29.5 (ranging from -3.2 to +82.7) µg/L in the Immunovac VP4 group, with this difference being statistically significant (p=0.005). It should also be noted that at baseline nasal sIgA levels in both COVID- 19 patients were higher than healthy unvaccinated study subjects (p<0.001 for comparisons of the values in both study to the median reference value [29.9 µg/L]), 30 days after the start of treatment in the control group this parameter was similar to that in healthy subjects (p=0.40) while in the Immunovac VP4 group it remained higher than in healthy subjects (p<0.001) (Figure 3).
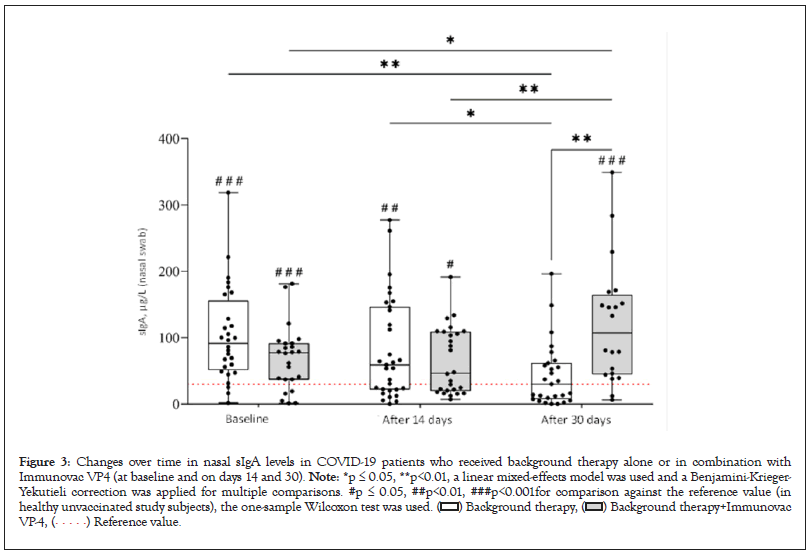
Figure 3: Changes over time in nasal sIgA levels in COVID-19 patients who received background therapy alone or in combination with
Immunovac VP4 (at baseline and on days 14 and 30). Note: *p ≤ 0.05, **p<0.01, a linear mixed-effects model was used and a Benjamini-Krieger-
Yekutieli correction was applied for multiple comparisons. #p ≤ 0.05, ##p<0.01, ###p<0.001for comparison against the reference value (in
healthy unvaccinated study subjects), the one-sample Wilcoxon test was used. 
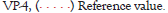
At baseline COVID-19 patients had high CRP levels, which were comparable in the study groups (p=0.15). On day 5, CRP levels showed a statistically insignificant decrease from baseline in the control group (from 2.0 [0.3–5.5] mg/L to 1.6 [0.1–6.5] mg/L (p=0.76) and a statistically significant reduction form baseline in the Immunovac VP4 group (from 4.3 [0.7–8.8] mg/L to 0.3 (0.2–5.8) mg/L (p=0.004). On day 5 the delta (change from baseline) of CPR was -3 (ranging from -7.1 to -0.4) mg/L in the Immunovac VP4 group vs. -0.1 (ranging from -0.9 to 3.9) mg/L in the control group (p=0.01) (Figure 4).
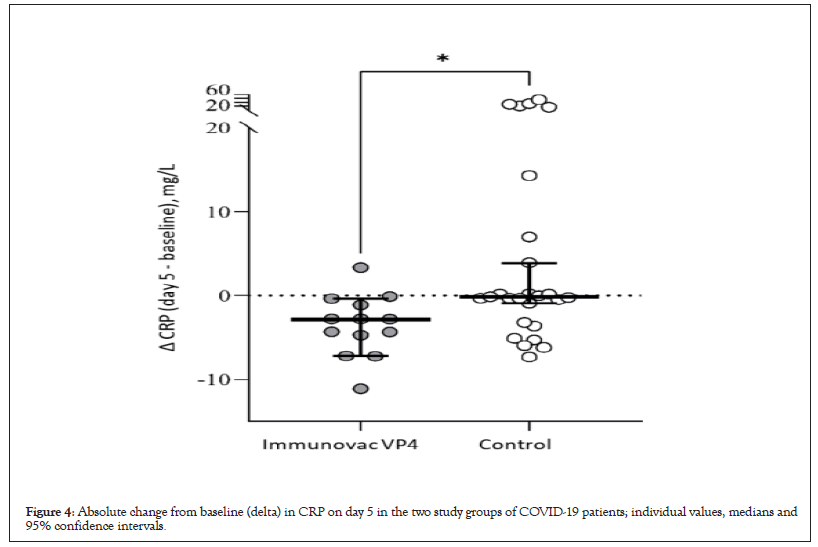
Figure 4: Absolute change from baseline (delta) in CRP on day 5 in the two study groups of COVID-19 patients; individual values, medians and 95% confidence intervals.
Analysis of the clinical efficacy of Immunovac VP4 as part of a combination treatment for patients with moderate COVID-19-associated lung disease revealed certain differences. The median duration of fever was estimated using the Kaplan-Meier test. In the Immunovac VP4 group fever persisted for a shorter period than in the control group: 1 (0.5–2) day vs. 4 (1–7) days (p=0.002) (Figure 5).
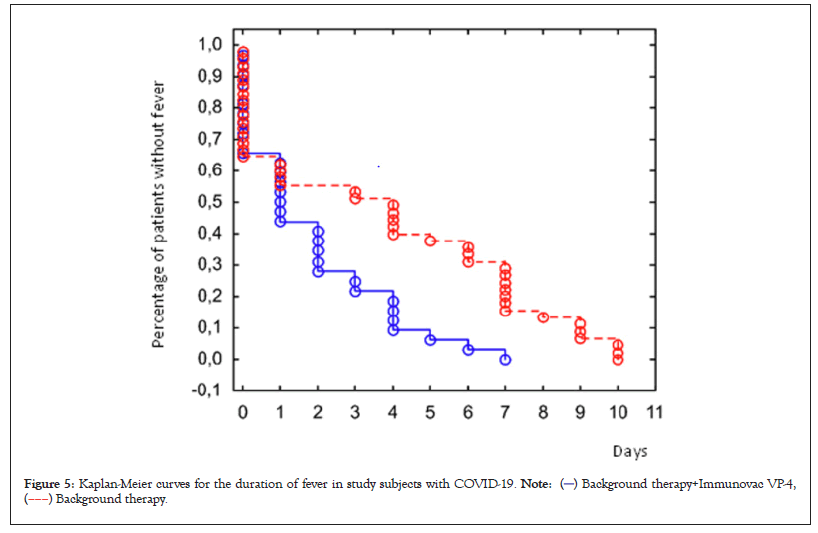
Figure 5: Kaplan-Meier curves for the duration of fever in study subjects with COVID-19. 
The duration of hospital stay was also shorter in the Immunovac VP4 group than in the control group: 16 (13-19) days vs.19 (16-22) days, p=0.03.
Assessment of Immunovac VP4 tolerability showed that after subcutaneous administration a red area (hyperemia) was observed at the injection site in 16 patients; it measured 2.5–5.0 cm in diameter in 10 subjects (31.2%) and 5.1–10.0 cm in diameter in 6 patients (18.8%) and did not persist for more than 3 days. There were no reported local infiltrates caused by injections. Systemic reactions in the Immunovac VP4 group included only fever of 38–38.4°С in three patients (9,3%) and 38.5–38.9°С in one patient (3.1%), which was similar to the rates in the control group (9.3% and 3.1%, respectively). Fever did not persist for longer than one day and did not require any medication treatment as well as in case of local reactions. Intranasal administration of Immunovac VP4 was not associated with any local mucosal reactions in the upper airways.
The 2009-2010 pandemic caused by the pandemic influenza strain gave a new impetus to the development of new adjuvant vaccines. The next pandemic caused by the spread of SARS-CoV-2 reignited research efforts to develop mucosal vaccines. Studies on murine models expressing human ACE2 showed that intranasal ChAd-SARS-CoV-2 vaccine induces high levels of neutralizing antibodies, promotes systemic and local IgA and T-cell responses and almost entirely prevents SARS-CoV-2 infection in both the upper and lower respiratory tracts [25-27]. Immunization at mucosal sites is believed to provide a better virus clearance from the airways and thus prevent its transmission [28]. Therefore, in the future immuno-biological medications that activate mucosal immunity can be viewed as a promising tool for the prevention of respiratory infections.
A new research direction could involve developing immune-active agents to be used in the phase of active inflammation, when SARS-CoV-2 begins spreading systemically. For example, vaccines containing bacterial ligands have long been used to restore mucosal immune function and thus to prevent complications of respiratory infections [29-34]. Consequently, acting as natural immuno-modulatory agents, bacterial vaccines not only induce impaired innate and adaptive immunity, but also suppress excessive immune reactions.
In our study, salivary sIgA levels remained high throughout the active phase when the patients received treatment and within two weeks following discharge (on day 30 of the study) and did not differ from the values seen on admission. It means that despite the cessation of SARS-CoV-2 shedding, improvement in the course of the disease and amelioration in clinical signs, inflammation was not fully resolved. Saliva is a composite biomarker which not only reflects the state of local immunity (as sIgA levels which we focus on in our studies), but also helps to assess a systemic immune response [35].
Pharyngeal sIgA levels in COVID-19 patients were similar to those in healthy subjects with no history of SARS-CoV-2 infection or exposure to SARS-CoV-2 both in the active phase of the disease and after discharge from hospital. So, we did not observe any changes in sIgA levels in the inflammatory phase, however, the levels of these immunoglobulin’s changed in patients who were immunized with the vaccine containing bacterial ligands. In this study group, sIgA levels measured on day 30 of the study were higher than in patients receiving only background therapy and even those in healthy subjects; they were also higher than at baseline, i.e. on admission to hospital. Therefore, using the Immunovac VP4 vaccine as part of a combination treatment for COVID-19 patients promotes the production of sIgA in the pharyngeal compartment, which will later highly likely decrease the susceptibility to other respiratory pathogens.
An evident trend was observed in nasal sIgA levels which were high at baseline and by day 30 gradually returned to normal, i.e. the levels seen in healthy subjects with no history of exposure to SARS-CoV-2. In contrast, in the patients receiving Immunovac VP4 in combination with background therapy, nasal sIgA levels increased by day 14 and on day 30 still remained higher than at baseline, which was an indirect sign of an activated production of sIgA in the nasal compartment.
Thus, the convalescence phase of moderate COVID-19 was associated with particular changes in sIgA levels in the compartments we focused on in our study. The assessment of tolerability of Immunovac VP4 used as part of a combination treatment for patients with moderate COVID-19-associated lung disease showed that its administration from day 1 to day 11 of hospital stay was associated with only local injection-site reactions (skin hyperemia that resolved without any medication treatment) in 50% of the patients. It was impossible to assess the rate of systemic reactions because the rates of fever were similar (12.4%) in the patients who received and did not receive Immunovac VP4 as part of a combination treatment regimen.
The assessment of clinical efficacy of Immunovac VP4 showed that in the Immunovac VP4 group, the patients experienced a more significant reduction in inflammation on day 5, as seen by a statistically significant decrease in CRP and the resulting reduction in the duration of fever and the length of hospital stay.
Therefore, using the Immunovac VP4 vaccine as part of a combination treatment for COVID-19 patients promotes the production of sIgA in the pharyngeal and nasal compartments and improves the clinical course of the disease, which may later influence the susceptibility to other respiratory pathogens [35,36].
Our study showed that on admission to hospital patients with moderate COVID-19 had high secretory IgA levels in biological samples taken from different compartments of the upper respiratory tract, i.e. epithelial cells of the nasal mucosa and salivary gland secretions. Thirty days after the start of treatment, i.e. two weeks following discharge from hospital, nasal sIgA levels reached those observed in the healthy subjects with no history of COVID-19 or exposure to SARS-CoV-2, while sIgA levels in secretory gland secretions did not change throughout the study. Pharyngeal sIgA levels in COVID-19 patients did not differ from those in healthy subjects at any study time point.
Using an immuno-stimulant agent containing bacterial ligands (Immunovac VP4) as part of a combination treatment for COVID-19 patients is associated with a gradual increase in the production of sIgA in the nasal and pharyngeal compartments compared to its baseline intensity, which accounts for peaked levels of these immunoglobulins at week 2 after discharge from hospital. It also reduces the CRP level and shortens the duration of fever and the time to recovery. It cannot be excluded that the activation of the sIgA production in these compartments of the respiratory tract is accompanied by other changes in mucosal as well as systemic immunity.
The authors declare that there is no conflict of interest.
The authors received no specific funding for this work.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Kostinov M, Svitich O, Chuchalin A, Osipcov V, Khromova E, Abramova N, et al. (2023) Secretory IgA and Course of COVID-19 in Patients Receiving a Bacteria-Based Immunostimulant Agent in Addition to Background Therapy. J Clin Cell Immunol.14:682.
Received: 08-Feb-2023, Manuscript No. JCCI-23-21751; Editor assigned: 10-Feb-2023, Pre QC No. JCCI-23-21751 (PQ); Reviewed: 24-Feb-2023, QC No. JCCI-23-21751; Revised: 03-Mar-2023, Manuscript No. JCCI-23-21751 (R); Published: 13-Mar-2023 , DOI: 10.35248/2155-9899.23.14.682
Copyright: © 2023 Kostinov M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.