Research Article - (2017) Volume 3, Issue 2
Sediment Treatment for Increasing Ph and Reducing Heavy Metal Cadmium (Cd) In Acid Mine Drainage
*Corresponding Author: Fahruddin, Faculty of Mathematics and Natural Sciences, Department of Biology, Hasanuddin University, Makassar, Indonesia, Tel: 62-85276985047 Email:
Abstract
Acid mine drainage could be treated by using wetland sediment as the source of sulfate-reducing bacteria for increasing pH and reducing heavy metal. The purpose of this study is to compare between the abilities of mangrove and swamp sediments in increasing pH and reducing heavy metal cadmium in acid mine drainage. Acid mine drainage experiment was conducted in a reactor by adding sediment (20%) and compost (10%), then incubated for 30 days. The pH changes were measured using pH meter, while the cadmium content was analyzed using Atomic Absorption Spectrophotometry (AAS), both was measured on the fifth day. The result showed that swamp sediment increased pH to 6.8, while mangrove sediment increased pH to 6.2 at the end of incubation. The results for cadmium reduction also indicated that swamp sediment (P2) could reduce cadmium from 1.88 to 0.17 ppm (90.96%), while mangrove sediment (P1) reduced cadmium from 1.72 to 0.24 ppm (86%). In addition, treatment only with compost (P3) and treatment without sediment and compost to control (P4) did not show any significant changes both for pH increase meant or cadmium reducement.
Keywords: Sediment; Acid mine drainage; Cadmium
Introduction
The advancement of mining industries in Indonesia is accompanied by the increasing of environmental issues, especially on the aquatic environment as the consequences of acid mine drainage. Acid mine drainage pollution could affect the life of flora fauna, as well as human health [1,2]. Mainly, acid mine drainage is formed from the exposure of sulfite minerals in the form of metal sulfite, then oxidized into sulfate, and would form acid liquid if it exposed to water. Acid mine drainage has a low pH range (3-4), so it could dissolve heavy metals [3-5]. Therefore, it could be said that acid mine drainage is a hazardous waste from mining activities due to its acidic character that could dissolve heavy metal and is toxic to humans [6]. One of harmful heavy metal that commonly found in mining waste is cadmium, especially in lead or zinc mining. Cadmium usually found in a form of mineral sphalerite (ZnS) [7].
Acid mine drainage needs to be well managed in order to change it into the harmless material when entering water bodies, such as river or lake. Treatment of acid mine drainage is commonly conducted by applying chemical substances, such as limestone, or treated physically by immersing it into a big hole. However, both of those treatments are known to be inefficient, not eco-friendly, and expensive [3,8].
Due to some treatment problems, biological treatment or bioremediation that utilizing microorganism, such as sulfate-reducing bacteria, become a good alternative for treating acid mine drainage. The advantages of biological treatment are eco-friendly, efficient, and continuously developed to be better on treating mining liquid wasted [6].
Sulfate-reducing bacteria are capable of reducing sulfate and heavy metal ion concentration in acid mine drainage [4,5]. These bacteria are commonly found in the muddy substrates, such as wetland sediment. The condition of wetland sediment is anaerobic and rich with organic materials that supported many kinds of sulfate-reducing bacteria, so that wetland sediment naturally treats sulfite contaminants [9,10].
The utilization of sediment as the inoculum sources for sulfatereducing bacteria could be applied in a bioreactor because bacteria naturally stay in the wetland sediment [11].
The experiments of [10,12] found that the addition of swamp sediment in acid mine drainage could reduce the sulfate concentration and increase the pH. The activity of sulfate-reducing bacteria is in contrary with Thiobacillus ferrooxidant that triggers the formation of acid mine drainage. Therefore, according to the explanation above, we focused on comparing mangrove and swamp sediment as the inoculum sources for sulfate-reducing bacteria for elevating pH and reducing cadmium in acid mine drainage.
Materials and Methods
Samples
Materials used in this study were acid mine drainage, collected from mangrove and swamp sediments, and compost.
Methods
Sample collection: A sample of acid mine drainage was obtained from a mining in Camba, Bone Regency. Swamp sediment was collected around Makassar city, Indonesia, while mangrove sediment was collected from Tallo mangrove forest, Makassar. The sample was put in a tight head plastic drum and kept in a refrigerator at 2ºC. Compost was obtained from the seller of decorative plants on Panaikang, the Makassar city.
Acid mine drainage and sediment characterization: Acid mine drainage sample was characterized by its pH using pH meter [13]. The sediment and compost were characterized based on total organic carbon using TOC meter [14] and nitrogen content using Micro Kjeldahl method [15].
Treatments: The treatments of acid mine drainage with sediments were conducted in a column of the anaerobic bioreactor. The processing column was equipped with wire frame at the bottom part that serves as a barrier between sediment and compost. Acid mine drainage was gradually poured into the column. The treatment was prepared using 3 liters of acid mine drainage with details of treatment: mangrove sediment 20% and compost 10% (P1); swamp sediment 20% and compost 10% (P2); compost 10% (P3); and control or without sediment and compost (P4). Each treatment with duplicate were used and incubated for 30 days at room temperature. During the incubation period, pH and cadmium content measurements were conducted every fifth day.
pH measurement: pH was measured using pH meter. pH meter was first calibrated in buffer solution pH 7, then activated until stabilized for about 15-30 min. The electrode was then rinsed with distilled water and dried. Next, the electrode was dipped into the acid mine drainage until resulting a stable output.
Cadmium (Cd) analysis: Two ml acid mine drainage sample were placed in a 100 ml chemical glass, to which 10 ml concentrated nitric acid were added, mixed and heated until dissolved. The acid residue was evaporated, rinsed with distilled water through the glass wall, filtered with whatman 42 filter paper in a volumetric flask 100 mL at pH 2, while the excess acid was neutralized by adding NaOH 6 M and then, the absorbance was measured using AAS with A varian model AA-1275 flame and a deuterium background corrector was used for determination of Cd ions. The wavelength was set at 228.8 nm resonance line and the spectral band pass at 0.5 nm. A standard solution of Cd with gradual concentrations to aliquots of the 1:10 diluted sample solution. The cadmium concentration according to the linear equations. Results of cadmium analysis were reported in ppm units.
Results and Discussion
The sediment of mangrove and swamp were used as the inoculum source of sulfate-reducing bacteria for treating acid mine drainage due to its species richness of sulfate-reducing bacteria [11,16]. On the other hand, compost that is rich with organic matter becomes the carbon sources for microorganism development. Before starting the acid mine drainage treatment, preliminary analyses for the content of organic carbon, total phosphorus, total nitrogen, and cadmium concentration were conducted in order to determine the initial condition of each sediment and compost. The preliminary analysis is presented in Table 1. The result showed that both mangrove and swamp sediments were already contained cadmium at its initial condition, approximately 0.001 ppm and 0.003 ppm respectively, while compost did not contain cadmium. The presence of cadmium was caused by the sampling location that located near the settlement area and household industry. In addition, [17] stated that cadmium could be presented through a natural process in a sediment.
| Sediment types | C Organic | N Total | P Total |
|---|---|---|---|
| Swamp sediment | 32.6% | 0.42% | 0.26% |
| Mangrove sediment | 29.7% | 0.51% | 0.33% |
| Compost | 22% | 13% | 6% |
Table 1. Content of carbon, phosphorus and nitrogen of sediments and compost samples.
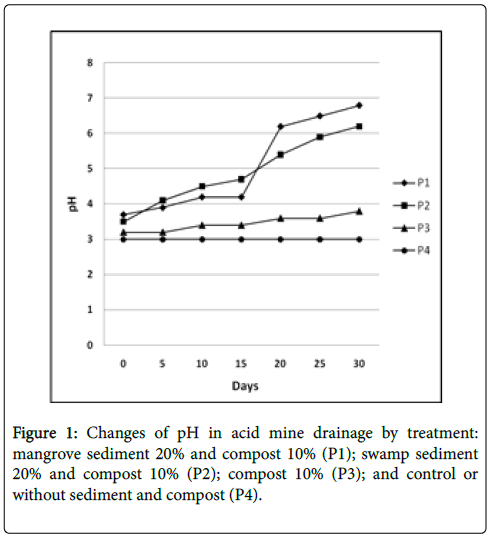
The result of pH changes in acid mine drainage affected by sediment treatment is presented in Figure 1. The P1 treatment demonstrated a slow pH increase that started in day 10 to day 30, from pH 3.5 to pH 6.2. Meanwhile, P2 treatment demonstrated a rapid increase in pH value from pH 3.7 into pH 6.2 in day 20, then pH 6.5 in day 25, and finally pH 6.8 in day 30. The P3 treatment with pH 3.2 represented a slight increase of pH value into pH 3.8 from day 25 to 30. The P4 treatment showed no changes in its pH.
Koschorreck [18] explained that the pH increase in acid mine drainage treated with sediment is occurred because of the decrease in sulfate concentration as the result of sulfate-reducing bacteria activities. Basically, sulfate receives electron that resulted in sulfate reduction to sulfide and causes decreasing in sulfate concentration.
Church [19] suggested that the sulfate reduction process by the sulfate-reducing bacteria group produces sulfide and bicarbonate that affected the rise in pH. Therefore, sulfide would react with dissolved metal ions to form dissolved metal sulfide [20]. The result of this study demonstrated pH increase from acid into neutral in acid mine drainage treated with mangrove and swamp sediment. A low pH of acid mine drainage is caused by the reaction between sulfide minerals and water that resulted in the release of metal and hydrogen ions, meanwhile, sulfide ions were oxidized into dissolved sulfate ions and H+ ions would lower the pH [21,22].
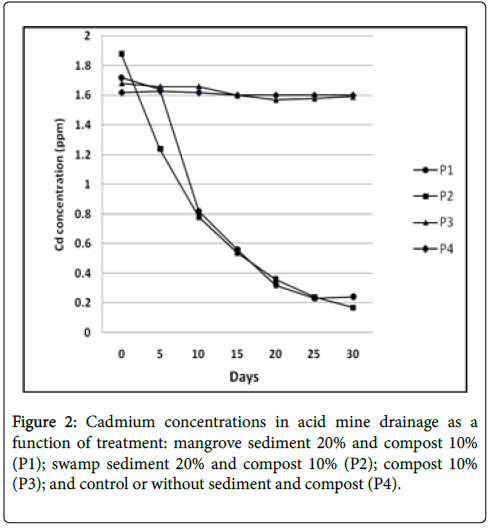
The analysis result of cadmium content in acid mine drainage that treated with sediment is presented in Figure 2. The cadmium concentration in P1 treatment started to reduce from day 10 to day 30, where the initial concentration was 1.72 ppm and was reduced to 0.24 ppm at 86% reduction. The P2 treatment showed more rapid reduction that started from 1.88 ppm, went to 1.24 ppm on day 5 and finally reached to 0.17 ppm on day 30, at 90.96% reduction.
The P3 treatment demonstrated lower cadmium reduction compared to P1 from 1.68 to 1.59 ppm or 5.36% reduction. On the other hand, the P4 treatment showed slight differences between the first day and the last day, being 1.62 ppm and 1.60 ppm, respectively, representing 1.23% reduction. The result showed that P1 treatment with addition of mangrove sediment resulted in more effective cadmium reduction compared to P2 treatment with addition of swamp sediment. These results correspond to the vegetation that grows on the surface and influences the organic matter content, sediment texture, and a number of microbes [23].
According to the study result, the reduction of cadmium concentration in acid mine drainage treated with mangrove or swamp sediments demonstrated that the activity of sulfate-reducing bacteria in both sediments are capable of reducing heavy metal ions of cadmium and resulted in deposited cadmium metal ions [24].
The mechanism of cadmium metal ion reduction in acid mine drainage is started with the sulfate reduction (SO4) into sulfide, then reacted with metal cation and formed metal sulfide that is deposited at the bottom of the bioreactor [9,25]. Therefore, the sulfate reduction process produces H2S that has a role as an electron donor and reduce metal cation into metal sulfide. Low cadmium reduction presented in P3 and P4 treatments occurred because of evaporation and deposition process from loss abiotic factor process [1].
Conclusion
According to the study results, it could be concluded that treatments with mangrove and swamp sediment could increase the pH value of acid mine drainage, reaching to 6.8 and 6.2 at the end of experiment period, respectively. Meanwhile, treatment without sediment showed no detectable change in pH during the incubation. On the other hand, swamp sediment could reduce the cadmium concentration in acid mine drainage by 90.96%, while mangrove sediment reduced it by 86.05%. Treatment without the addition of sediment showed almost no reduction in cadmium concentration.
Acknowledgement
The author would like to thank the Director General of Higher Education and Technology Research for research assistance for the 2016 budget and to the Hasanuddin University Research Institute on the implementation of this research.
References
- Gaikwad RW, Sapskal R (2011) Acid Mine Drainage: A Water Pollution Issue In Mining Industry. International Journal of Advanced Engineering Technology 4: 19-21.
- Fahruddin, As’adi A (2015) Use Of Organic Materials Wetland To Improving The Capacity Sulfate Reduction Bacteria (SRB) of Reduce Sulfate in Acid Mine Water (AMW). Asian Jr of Microbiol Biotech Env Sc 17: 1-4.
- Dold B (2010) Basic concepts in environmental geochemistry of sulfidic mine-waste management. Waste management 173-198.
- Burgos WD, Borch T, Troyer LD, Luan F, Larson LN, et al. (2012) Schwertmannite and Fe oxides formed by biological low-pH Fe(II) oxidation versus abiotic neutralization: impact on trace metal sequestration. Geochim Cosmochim Acta 76: 29-44.
- Hedrich S, Johnson DB (2012) A modular continuous flow reactor system for the selective bio-oxidation of iron and precipitation of schwertmannite from mine-impacted waters. Bioresour Technol 106: 44-49.
- Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338: 3-14.
- Stanton MR (2005) Baseline Laboratory Studies of Sphalerite (ZnS) Dissolution: Effects on Aqueous Metal Concentrations and Solubilization Rates. National Meeting of the American Society of Mining and Reclamation, June 19-23, 2005. Published by ASMR, Montavesta Rd., Lexington, Kentucky.
- Hards S, Higgins JP (2004) Bioremediation of Acid Rock Drainage Using Sulfate Reducing Bacteria. Jacques Whit Environment Limited. Ontario.
- Lovley DR, Elizabeth JPP (1986) Organic Matter Mineralization with Reduction of Ferric Iron in Anaerobic Sediments. Applied and Environmental Microbiology 51: 683-689.
- Sanchez-Andrea I, Nuria R, Ricardo A, Jose LS (2011) Microbial Diversity in Anaerobic Sediments at Río Tinto, a Naturally Acidic Environment with a High Heavy Metal Content. Appl Environ Microbiol 77: 6085-6093.
- Fukui M, Susumu T (1996) Microdistribution of sulfate-reducing bacteria in sediments of a hypertrophic lake and their response to the addition of organic matter. Ecological Research 11: 257-267.
- Pester M, Knorr KH, Friedrich MW, Wagner M, Loy A (2012) Sulfate-Reducing Microorganisms in Wetlands-Fameless Actors in Carbon Cycling and Climate Change. Front Microbiol 3: 72.
- Greenberg AE, Clesceri LS, Eaton AD (1992) Standard Methods for the Examination of Water and Waste Water (18th ed) Public Health Association, Washington, DC: American.
- Nur MA (1989) Chemical analysis, Inter-University Center-Biotechnology, Bogor Agricultural University, Bogor.
- Black CA, Evans DD, White JL, Ensminger LE, Clarck FE, et al. (1965) Methods of Soil Analysis. American Society of Agronomy, Inc. Wisconsin.
- Coleman ML, David BH, Derek RL, David W, Kenneth P (1993) Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature 361: 436-438.
- Bernhoft BA (2013) Cadmium Toxicity and Treatment. The Scientific World Journal 2013: 1-7.
- Koschorreck M (2008) Microbial sulfate reduction at a low pH. FEMS Microbiol Ecol 64: 329-342.
- Church CD, Richard TW, Charles NA, Robert OR, McCleskey RB (2007) Microbial sulfate reduction and metal attenuation in pH 4 acid mine water. Geochem Trans 8: 8-10.
- Voordouw G (1995) The Genus Desulvibrio: Centennial. Appl Environ Microbiol 61: 2813-2819.
- Germida JJ (1998) Transformation of Sulfur. Principles and Application of Soil. Microbiology Prentice Hall. New Jersey.
- Fahruddin, Nurhaedar A, Nursia L (2014) Comparison of The Capacity of Swamp and Rice Fields Sediment to Reduce Sulphate in Acid Mine Water (AMW). Jurnal Sainsmat 3: 135-142.
- Fahruddin (2005) Evaluation of CO2, CH4, and N2O emissions in biodegradation of Oil Sludge Throught Landfarming. Research Report of Research Grant FY 2004-2005: Osaka Gas of International Cultural Exchange. Environmental Research Center, Bogor Agricultural University. Bogor.
- Suyasa IWB (2002) Increased pH and Dissolved of Heavy Metal of Acid Mine Drainage With Sulfate Reducing Bacteria from the Black Water Ecosystem at Borneo, Ph.D. Dissertation, Bogor Agricultural University, Bogor.
- Al-Zuhair S, Muftah HE, Huda A (2008) Sulfate inhibition effect on sulfate reducing bacteri. J Biochem Tech 1: 39-44.
Copyright: © 2017 Fahruddin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.