Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Research Article - (2008) Volume 1, Issue 8
Drug induced toxicities account for nearly half of the cases of acute liver failure in adults over age 50 in the US. Acetaminophen (APAP) is a commonly used non-prescription drug that can be purchased in drug stores and supermarkets. Cases of unintentional APAP overdose and associated hepatotoxicity have been reported to the FDA. Early detection of hepatotoxicity caused by APAP is of great interest to both the scientific and public communities. Therefore, in this study SELDI based analysis of mouse serum following APAP administration was conducted to demonstrate the feasibility of identifying hepatic toxicity biomarkers from a readily available body fluid. Late hepatotoxicity caused by APAP was detected by SELDI. Furthermore, early stage hepatotoxicity caused by APAP that was not detected pathologically was clearly discernable using SELDI. Our results suggest that SELDI analysis can be used for early detection of hepatotoxicity based on the expression pattern of the biomarker peaks.
Keywords: Hepatotoxicity, Acetaminophen, SELDI, Biomarker, Serum.
APAP, acetaminophen; SELDI-TOF, Surface-Enhanced Laser Desorption Ionization-Time-Of-Flight; MALDI-TOF, Matrix-Assisted Laser Desorption Ionization-Time-Of-Flight; MS, mass spectroscopy; 2D-GE, two-dimensional gel electrophoresis; SPA, sinapinic acid; PCA, principal component analysis; WCX, weak cation exchange; TIC, total ion current; ALT, alanine transaminase; AST, aspartate transaminase
Hepatotoxicity is the number one reason for drug recall and hence it is of concern to the FDA, to pharmaceutical companies, and to consumers (Arundel et al., 2007; Mumoli et al., 2006; Lee, 2003; Boyd et al., 1966). Drug-induced toxicities account for nearly half of the cases of acute liver failure in adults over age 50 in the US. Approximately 25% of these drug-induced toxicities may be due to acetaminophen (APAP). APAP is potentially involved in a large percentage of adverse drug responses and can result in acute liver failure leading to death or liver transplantation (Holubek et al., 2005; FDA, 2002).
APAP is a widely available non-prescription drug that is used for the treatment of pain and fever. Cases of unintentional APAP overdose and associated hepatotoxicity have been reported to the FDA and additional adverse events have been reported in the medical literature (Andrade et al., 2007; Larson et al., 2005; James et al., 2003; Boyer et al., 1971; Mitchell et al., 1973a; Mitchell et al., 1973b). Thus, prediction or early detection of hepatotoxicity caused by APAP is of great concern to both the scientific and public communities.
Biomarkersof exposure, effect, toxicity, and susceptibility can be observed following exposure to compounds (Timbrell, 1998). These may include markers of liver injury, liver function tests, and markers of recovery (Amacher, 2002). The ability to discriminate between the toxicity of agents and their pharmacological actions is critical for the future direction of toxicology. The development of biomarkers in an accessible biofluid to understand the drug response continuum in susceptible organs is essential. In addition, the development of biomarkers that signal early but significant adverse effects from exposure to drugs could prevent future, severe health consequences. In clinical trials, serum levels of hepatic enzymes are measured as indicators of liver injury, but more specific biomarkers of drug induced hepatic injury are needed. Specific, sensitive, and predictive biomarkers associated with the induction of liver toxicity and that have the potential to predict progression to fulminant liver failure need to be identified.
The development of biomarkers of drug toxicity and safety have attracted attention from both the pharmaceutical industry and regulatory agencies (Goodsaid, 2004; Goldber et al., 2002; Petricoin et al., 2004a; Petricoin et al., 2004b). Liver enzyme levels in serum, indicative of hepatocellular damage, have been used as biomarkers for preclinical and clinical drug safety evaluation (Clarke et al., 1997). The efforts to discover safety biomarkers for toxicity detection makes it clear that reliable biomarkers for early detection of toxicity should ideally be easily measured in easily accessible tissues for both preclinical and clinical tests. Such markers have been described that are apparently specific and sensitive indicators of tissue damage, albeit not necessarily predictive (Wetmore et al., 2004; Petricoin et al., 2004b).
The purpose of this study was to focus on predictive and diagnostic biomarkers of hepatotoxicity, thus emphasizing potential indicators of liver injury.
SELDI-TOF MS is a variation of MALDI-TOF MS that combines TOF with special chips, which can be modified to bind required classes of proteins or peptides. Highly specific proteins or peptides can be immobilized on a specific chip. Thus, it acts like a separation step for selection of specific proteins or peptides. But MALDI-TOF MS needs a separation process and a digestions enzyme. Furthermore, SELDI performs excellently in separating low molecular weight proteins. 2D-GE is not efficient for this purpose. Another advantage of SELDI over MALDI and 2D-GE is its high thorough-put ability. The SELDI method can simultaneously detect the relative expression levels of numerous proteins in biological fluid and thus has the potential to be suitable for the identification of toxicity biomarkers (Dare et al., 2002; Chu et al., 2002; Xiao et al., 2004; Gineste et al., 2003).
The focus of the present study is the detection of predictive biomarkers of liver injury in the mouse, using exposure to APAP as an example. We examined SELDI-TOF MS based proteomic analysis as a method of developing predictive and diagnostic biomarkers of acute liver injury from readily accessible serum samples. Our results revealed that late hepatotoxicity caused by APAP exposure can be detected using SELDI-TOF MS. Furthermore, the early hepatotoxicity caused by APAP that was not detected histopathologically was clearly discernable using SELDI-TOF MS. It was also demonstrated that sample fractionation increases the ability to identify predictive and diagnostic biomarkers through SELDI-TOF MS analysis.
Sample Treatment for SELDI Analysis
To discover serum biomarkers associated with liver toxicity, SELDI based proteomic analysis was performed on serum samples obtained from mice treated with APAP. Fourteen male C57/BL mice were treated with APAP at 300 mg/kg IP. Serum samples were obtained at 0.5 hr (6 mice), 4.0 hr (4 mice), and 24.0 hr (4 mice) after dosing. Control serum samples were obtained from 4 mice that received saline injections IP. All mice were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). Biochemical assessment of toxicity was measured through assays for aspartate transaminase (AST) and alanine transaminase (ALT) using commercially available kits (Sigma).
Both non-fractionated and fractionated serum samples were analyzed using the CM10 weak cation exchange ProteinChip arrays (Ciphergen Biosystems, Inc., Fremont, CA) suggested by (Fung et al ., 2000). Fractionated samples were subjected to anion exchange chromatography with stepwise pH elution into six fractions. Briefly, serum (20 µl) was centrifuged for 10 minutes and then mixed with 30 ml chaotropic U9 denaturing and solubilization buffer (9 M urea, 2% CHAPS, 50 µM Tris-HCL, pH 9) for 30 minutes. Sample fractionation was performed on a Q HyperD F resin plate (180 µl resin) (Ciphergen, Fremont, CA). The plate was prewashed and equilibrated with U1 solution (1 M urea, 0.2% CHAPS, 50 mM Tris-HCL, pH 9) prior to the addition of samples to the 96 well fractionation plate. The anion exchange fractionation included the following elution steps: (1) 50 µM Tris-HCL, 0.1% OGP (a nonionic detergent, b-D-glucopyranoside), pH 9; (2) 50 µM HEPES, 0.1% OGP, pH 7; (3) 100 µM Na Acetate, 0.1% OGP, pH 5; (4) 100 mM Na Acetate, 0.1% OGP, pH 4; (5) 50 µM Na Citrate, 0.1% OGP, pH 3; and (6) 33.3% isopropanol, 16.7% acetonitrile, 0.1% TFA (trifluoroacetic acid). Each fractionated serum sample, as well as the non-fractionated serum, was spotted in duplicate on CM10 chips. A matrix of sinapinic acid (SPA) was added to each spot, and the chips were read at two appropriate laser energies to permit ionization and visualization of proteins below 20kD and those above 20kD, respectively.
Spectral Processing
Previously we have shown that the data examined in the present study were of high quality and did not show significant systematic variability between plates, chips or spots (Hong et al., 2005). The raw SELDI mass spectra were pre-processed prior to subsequent analysis of the expression profiles. Normalization by Total Ion Current (TIC) was applied to all spectra to minimize the variability in spectra obtained at different times using Ciphergen ProteinChip 3.2 software (Ciphergen Biosystems, Inc., Fremont, CA). The normalization process averaged the intensity used for all the spots, and adjusted the intensity scales so that all spectra could be displayed on the same scale. Baseline subtraction was conducted prior to normalization, as recommended by Ciphergen. Baseline subtraction offsets in the spectra were the result of both electrical noise and noise from the matrix (SPA). The lowest spectral amplitude was identified and was then used to correct the peak height and area. The Biomarker Wizard software application from Ciphergen was used to automatically detect the peaks present in all of the spectra. The spectral region from 0 to 2500 Da is unreliable for both normalization and peak detection due to matrix interference and was therefore not included in the analysis. A signal to noise ratio greater than 5 was used for the first pass selection of peaks, and a signal to noise ratio greater than 3 was used for the second pass. A cluster mass window of 0.3% was used. The presence of cluster sets in a minimum of 5% of samples was set because different protein expressions were expected in samples obtained from mice treated with APAP for varying time courses. Estimated peaks were added into the final profiles by the Biomarker Wizard application.
Data Analysis
Statistics were performed on groups of profiles for identification of potential biomarker proteins. Comparisons were examined between control mice and treated mice (at all time points) using the Biomarker Wizard software application. Intensity differences among the various groups of samples were calculated for all peaks (m/z positions). A peak was considered to have potential discriminatory power if statistically significant differences in its intensities were observed in all analyses (P ≤ 0.01). Principal component analysis (PCA) is widely employed in signal processing, statistics and neural computing to examine the maximum variability in highly dimensional data. Therefore, all possible combinations of peaks having potential discriminatory power in each comparison were tested by PCA using Spotfire DecisionSite 7.1 software (Somerville, MA, www.spotfire.com) to find a subset of protein peaks (potential biomarkers) best able to differentiate groups of samples.
Statistical analysis was performed on all peaks for groups of profiles for identification of biomarker proteins. The p values were generated with nonparametric tests from the Biomarker Wizard software application. Peaks with p values less than 0.01 were selected as the candidates for further analysis for identification of biomarkers. All possible combinations of the candidate peaks in each comparison group were analyzed using PCA to identify a subset of peaks having the most discriminatory power for the comparison.
Identification of Differentially Expressed Liver Toxicity-Associated Proteins in Serum of Mouse
Using SELDI-TOF mass spectroscopy proteomics technology (Ciphergen Biosystems), potential liver toxicity biomarker proteins differentially expressed in serum from mice at different time points after treatment with APAP were identified. A representative spectral view of specific candidate liver toxicity markers is shown in Fig. 1. The PCA results are listed in Table 1.
Figure 1: Examples of differentially expressed liver toxicity associated serum proteins. The representative SELDITOF mass spectra are from fraction 1 of control sample (C3), sample obtained 0.5 hr after APAP (A11), 4 hr after APAP (A3), and 24 hr after APAP (E8). Arrows at the bottom indicate the positions of biomarker plasma proteins. The expressions of both proteins 3333 Da and 6733 Da increased after treatment with APAP. The difference between control and 0.5 hr treatment was very small.
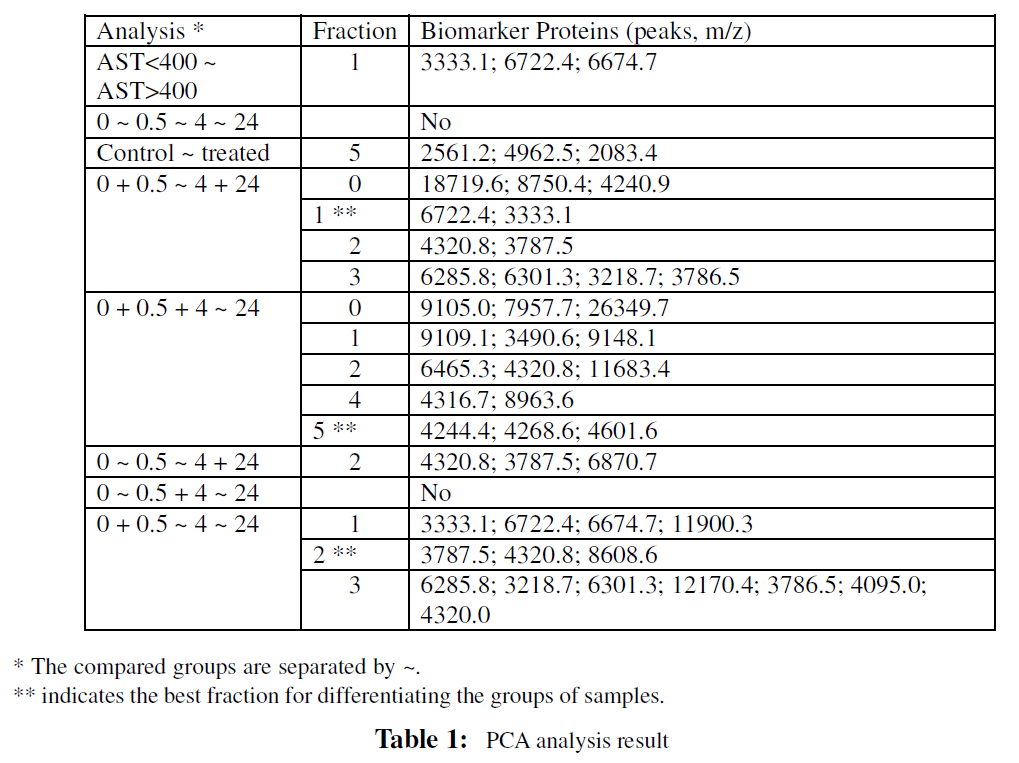
When comparing differences in the proteome between mice with AST levels less than 400 and mice with AST levels greater than 400, the peaks with m/z values of 3333.1, 6722.4, and 6674.7 in the SELDI spectra of fraction 1 were able to differentiate the two groups. No biomarker proteins (peaks in spectra) from other fractions or from the non-fractionated samples were found to differentiate the two groups of mice.
A unique set of biomarker proteins that could differentiate each of the treatment groups from the controls could not be found, either in the fractionated samples, or in the non-fractionated samples. Fraction 5 revealed peaks with m/z values of 2561.2, 4962.5, and 2083.4 that were identified as biomarkers to differentiate control mice from APAP treated mice.
Spectra from the non-fractionated serum with peak m/z values of 18719.6, 8750.4, and 4240.9 were found to differentiate the control plus 0.5 hr treatment groups from the 4 hr plus 24 hr treatment groups. Spectra from fractions 1, 2, and 3, with peak m/z values of 6722.4 and 3333.1, 4320.8 and 3787.5, and 6285.8, 6301.3, 3218.7, and 3786.5 respectively, were used to differentiate the two groups (control plus 0.5 hr versus 4 hr plus 24 hr). Differentiation of the two groups was most clearly seen in fraction 1.
The group of mice composed of the controls plus 0.5 hr and 4 hr after APAP exposure could be distinguished from the group of mice 24 hr after APAP exposure using either the spectra of (1) the non-fractionated samples (m/z values of peaks: 9105.0, 7957.7, 26349.7), (2) fraction 1 (m/z values of peaks: 9109.1, 3490.6), (3) fraction 2 (m/z values of peaks: 6465.3, 4320.8, 11683.4,), (4) fraction 4 (m/z values of peaks: 4316.7, 8963.6), and (5) fraction 5 (m/z values of peaks: 4244.4, 4268.6, 4601.6). The best separation of the groups was obtained by using the fraction 5 spectral peaks.
Only the peaks with m/z values of 4320.8, 3787.5, and 6870.7 in the spectra of fraction 2 were identified as able to separate the controls plus 0.5 hr treatment group from the 4 hr plus 24 hr treatment group.
Peaks with m/z values of 3333.1, 6722.4, 6674.7, and 11900.3 in the spectra of fraction 1, 3787.5, 4320.8, and 8608.6 of fraction 2, and 6285.8, 3218.7, 6301.3, 12170.4, 3786.5, 4095.0, and 4320.0 of fraction 3 were able to differentiate three groups of mice: the controls plus 0.5 hr treatment group; the 4 hr treatment group; and the 24 hr treatment group. Furthermore, fraction 2 was the best for the differentiation.
To summarize the PCA results: (1) There were no biomarkers (peaks) showing differential expression levels that distinguished all time points following treatment (2) biomarkers for treatment effect were detected in fraction 5 (3) protein expression patterns for the control plus the 0.5 hr treatment group were very different and could be easily differentiated from the four hr after treatment with APAPgroup (4) the protein expression pattern was changed even at 4 hr after treatment with APAP (5) the protein expression pattern at 0.5 hr is not only different from the controls, but also different from the pattern after longer treatment with APAP (4 hr and 24 hr after treatment).
Consistency between SELDI and Classical Pathology Experiment Results
Biochemical measures of toxicity (AST and ALT levels) are shown in Fig 2. As demonstrated, AST and ALT values were significantly increased at 4 and 24 hr after APAP. These findings are consistent with the SELDI analysis. Fig. 3 shows the PCA analysis of the SELDI-TOF mass spectra from fraction 2 using biomarker peaks at m/z values of 3878.5, 4320.8, and 8608.6. As shown in Fig. 3, the control samples and the samples from 0.5 hr treatment group (red points) differed from the 4 hr treatment (blue points) samples and from the 24 hr treatment group (yellow points).
Figure 3: Principal component analysis of SELDI spectra using biomarker peaks 3878.5, 4320.8, and 8608.6. The points represent the SELDI spectra from fraction 2 of the samples. Spectra of control samples and samples obtained from mice sacrificed at 0.5 hr after APAP were color coded in red; spectra of samples from mice sacrificed at 4 hr after APAP are shown in blue; spectra of samples from mice sacrificed at 24 hr after treatment are shown in yellow.
Identification of Biomarker Proteins for Early Liver Toxicity
Early toxicity detection is very important for protection. The traditional clinical chemistry measures did not show a statistically significant difference between the samples from 0.5 hr after treatment with APAP and the control samples (Fig. 2), consistent with previous data (James et al., 2005). SELDI analysis identified biomarker proteins after 4 hr treatment with APAP, consistent with the AST and ALT data. In addition, SELDI analysis also identified biomarkers predictive of early toxicity. The SELDI analysis on fraction 2 identified predictive biomarker peaks at m/z values of 2991.3, 6870.7, and 6577.4 that can differentiate early APAP treated samples (0.5 hr) from the control samples (PCA figure shown in Fig. 4.
Fractionation Enhances Biomarker Identification
Fractionation separates highly abundant proteins into a limited number of fractions, reducing signal suppression effects on lower abundance proteins. Moreover, fractionation prior to profiling increases the number of peaks detected and therefore increases the probability of novel biomarkers being identified. To compare the difference between fractionating and not fractionating, and demonstrate the utility of fractionation, all serum samples were assayed with and without fractionation using SELDI. Biomarker peaks diagnostic of liver toxicity (4 and 24 hr treatment groups) as differentiated from the control and 0.5 hr time point were identified from analysis of SELDI spectra of the non-fractionation samples. However, biomarker peaks identified from the analysis of SELDI spectra of the fractionation samples were better able to separate the same groups as shown in Fig. 5. The differences of AST and ALT levels between the group of samples at 4 hr and 24 hr treatment of APAP versus the group of samples from the control and 0.5 hr treatment of APAP are large. Therefore, biomarkers can be identified, independent of whether fractionation is applied. But, the differences of AST and ALT levels between samples at 4 hr after treatment with APAP and samples at 24 hr after treatment with APAP are much smaller (Fig. 2). Biomarkers that could separate the three groups (control plus 0.5 hours, 4 hours, and 24 hours) were not identified from non-fractionation samples analyzed by the SELDI spectra, consistent with the biochemical findings. Even the best subset of peaks was not able to separate the three groups well using non-fractionated samples (Fig. 6A), while fractionation of the same samples successfully separated these three groups of samples (Fig. 3). The levels of AST and ALT could not distinguish 0.5 hr APAP treatment from the controls (Fig. 2). Similarly, the best subset of peaks from the SELDI spectra of non-fractionated samples was unable to separate these samples as shown in Fig. 6B. The fractionation approach, however, resulted in predictive biomarkers that can distinguish the control samples from 0.5 hr treatment with APAP (Fig. 4). These results demonstrated that fractionation permits identification of predictive serum biomarkers that can not be identified without fractionation.
Figure 5: Comparison of principal component analysis of SELDI spectra of samples with and without fractionation. Panels B and D represent the SELDI spectra of non-fractionation samples. The points in A represent the SELDI spectra of fraction 1 of samples. The points in C represent the SELDI spectra of fraction 5 of samples. In A and B, control samples and 0.5 hr treatment samples were color coded in red and 4 hr and 24 hr treatment samples were color coded in blue. In C and D, control samples, 0.5 hr treatment samples, and 4 hr treatment samples were color coded in red and 24 hr treatment samples in blue.
Figure 6: Principal component analysis of SELDI spectra of non-fractionation samples. In A, spectra of control samples and samples of 0.5 hr treated with APAP were color coded in red spectra of 4 hr samples were in blue spectra of 24 hr samples were in yellow. In B, SELDI spectra of control samples were color coded in red spectra of 0.5 hr samples were coded in blue.
SELDI as a tool for high-throughput biomarker discovery in a variety of biological and clinical samples is growing rapidly and will no doubt contribute to an area in current proteomic research aimed at translating basic research into clinic practice. ProteinChip technology coupled with SELDI-TOF MS is an effective tool for the simultaneous detecting of the relative expression levels of proteins over a wide range of molecular weights in biological samples under different conditions. Using SELDI coupled with protein chip technologies for biomarker development is a complicated process that involves many steps. Thus, a high degree of variability of protein expression profiles from SELDI experiments occurs; and stringent quality control and assessment have to be applied to the experiments and subsequent data analysis. Previously we have examined the dataset described in the present study for quality by assaying control samples together with the study samples (Hong et al., 2005). All samples were randomly deposited on the protein chip spots followed by a QC/QA method developed in our laboratories. This process assures that the profiles generated from SELDI have a reasonable and acceptable quality and that the biomarkers identified from the profiles are reliable. It also ensures the biomarkers of hepatotoxicity identified in our present study are not confounding of systematical biases. Therefore, we believe that the reported results are reasonably robust and repeatable.
The global analysis of proteins permits a direct comparison of numerous potential disease markers in individuals that have a specific disease (e.g., hepatotoxicity or liver cancer) or who do not have the disease. Proteomic techniques permit a large number of proteins to be analyzed simultaneously for the detection of potential biomarkers. SELD-TOF mass spectrometric analysis is a proteomic technique that is based on the differential protein expression patterns observed between two types of samples. The utility of the SELDI approach lies in its strength for the simultaneous analysis of proteins and peptides with a molecular weight below 20kD. The parallel analysis of all of the proteins found in a sample permits the direct comparison between samples of two phenotypes (e.g., healthy individuals and cancer patients). This technique complements 2D gel electrophoresis approaches and has been used successfully to generate biomarkers for breast, prostate, ovarian, and bladder cancer (Issaq et al., 2002; Petricoin et al., 2002; Petricoin et al., 2004). SELDI analysis permits the separation, visualization, and molecular weight determination of proteins. However, identifying proteins corresponding to the peaks in the SELDI-TOF MS spectra is difficult and is the primary limitation of this approach.
One of the major obstacles in proteomic analysis of biological fluids is the presence of highly abundant proteins that can interfere with the resolution and sensitivity of proteome profiling techniques such as SELDI. In essence, to best analyze the content of a proteome, it must be fractionated into discrete groups of proteins followed by the separate analysis of each group. Fractionation of samples increases the total number of unique peptides and proteins that can be observed in SELDI-TOF mass spectra, as mass spectra may suffer from signal suppression due to the presence of proteins or peptides that are well-ionized and present in high amount. Moreover, excess levels of proteins, especially ones with high molecular weight, sometimes generate broader peaks because of distinct proteins of similar m/z. One protein or peptide may also have more than one peak in the mass spectra due to the presence of multiple charges. Recently, many studies have adopted fractionation prior to SELDI-TOF (Linke et al., 2004; Solassol et al., 2005). To evaluate the effect of fractionation prior to SELDI, both non-fractionation and fractionation of samples followed by SELDI, were investigated in this study. To increase the detection of proteins, as well as to alleviate signal suppression effects on low abundance proteins, serum samples were fractionated into six fractions followed by down-stream data analysis on each fraction for identification of biomarkers for both early (predictive) and late (diagnostic) hepatotoxicity. In the present study, fractionation resulted in the detection of biomarker peaks for early changes caused by APAP which can not be detected by traditional pathological testing. In addition, biomarkers for late hepatotoxicity were identified from both the fractionation and non-fractionation serum samples. These results indicate that fractionation of samples prior to SELDI analysis assists biomarker detection. Thus, although fractionation introduces time and complexity to sample treatment, fractionation is useful for biomarker detection in a complex matrix such as serum that contains many high abundance proteins.
We demonstrate the feasibility of SELDI-TOF MS for identifying hepatic toxicity biomarkers of mouse serum following APAP administration. Late hepatotoxicity caused by APAP was detected by profiling SELDI-TOF MS. Furthermore, early stage hepatotoxicity caused by APAP that was not detected pathologically was clearly discernable using SELDI-TOF MS. Our results suggest that SELDI analysis can be used for early detection of hepatotoxicity based on the expression pattern of the biomarker peaks.
Although this paper is a successful proof-of-principal study, it is important to keep in mind that the biomarkers of late and early stage hepatotoxicity identified using SELDI-TOF MS must be tested in validation. In future studies, we will investigate the relevance of these biomarker proteins and peptides in serum samples of rat and monkey following APAP administration. We could not address the biological function of these biomarkers in present study. Therefore, our future studies will also aim at defining the chemical characterization of these biomarkers by determining the peaks of biomarkers in SELDI-TOF MS using Tandem MS/MS technique.