Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2011) Volume 2, Issue 2
Solvent extraction of Copper (II) {Cu(II)} from aqueous solutions by organic solvent composing of lauric acid (extractant)/benzene (diluent) has been studied at T=298.2 K under atmospheric pressure. Effect of initial Cu(II) concentration in aqueous phase ( 5 X 10-4, 2.5 X 10-3, 5 X 10-3 and 2.5 X 10-2) M in pH=1.6 Sulphuric acid solution were investigated. Extraction was studied as a function of organic phase composition, acid concentration, aqueous pH, primary copper concentration. The copper concentrations were analysed by Spectroscopy. Percentage extraction (%E) of Cu(II) was studied. Distribution coefficient (D’) were measured to determine the extracting capability of the extractant. The distribution coefficient (D’) and extraction percentage (%E) increased with growth of pH. The results indicate pH, percentage extraction (%E), and distribution constant (D’) decrease with increasing initial Cu(II) concentration (5 X 10-4 to 2.5 X 10-2) M and they decrease with increase of lauric acid concentration (0.2 to 0.5) M.
Keywords: Extraction organic solvent; Cu(II); pH.
Various techniques remove Cu(II) from aqueous solutions, membrane filtration, flotation, electrolysis, biosorption, precipitation [4-5] and liquid- liquid extraction. Between various techniques extract Cu(II) from aqueous solutions, Liquid-liquid extraction is one of the effective techniques to extract Cu(II) from aqueous solutions [6]. So, liquid- liquid extraction is established technologic for recovery of metals from dilute aqueous product effluents [7-12]. In this work, Investigation reports on use solvent extraction in the separation of Cu(II) from aqueous solutions by lauric acid diluted in benzene under different experimental conditions: aqueous pH, Cu(II) and extractant concentration, and extract.
Materials
All the chemicals were used without further purification. Copper sulphate pentahydrate (CuSO4.5H2O) (Merck ≥ 99.6% purity), benzene (Merck ≥ 99% purity), lauric acid (probus ≥ 99% purity) sulphuric acid (H2SO4) (Probus R.A ≥ 98% purity) were purchased. Distilled and deionized water was used throughout all experiments. The structure of lauric acid is shown in I (Structure 1).
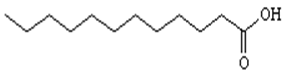
Extraction procedures
A volume of 10 ml of Cu(II) were prepared by dissolving appropriate amounts of CuSO4.5H2O (5 X 10-4, 2.5 X 10-3, 5X 10-3 and 2.5 X 10-2) M in distilled water loaded with 0.1M Na2SO4 and containing aqueous phase at 1:1 organic to aqueous volume ratio in glass cell. Organic phase was prepared with lauric acid (extractant) and benzene (diluents). A glass cell connected to a water thermostat was made to measure the liquid-liquid extraction data. The prepared mixtures were introduced into the extraction cell and were stirred for 2 h, and then left to settle for 2 h for phase separation. After being allowed to reach equilibrium, samples were carefully taken from each phase. Aqueous phase pH was measured with a Radiometer Copenhagen pH meter (model 62). The samples was used to 1.8 g/dm3 H2SO4, pH=1.6. The concentration of the Cu (II) in organic phase was obtained from a Hitachi UV-Vis Spectrophotometer (model 40-100) at a wavelength of 412 nm. All the experiments were carried out at constant temperature T=298.2 K. The temperature was estimated to be accurate to within solution ± 0.1K. The percentage extractions (%E) of Cu(II) were calculated according to [7-8]:
%E=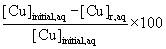 (1)
(1)
where [Cu]initial,aq is the initial Cu(II) concentration in the aqueous phase and [Cu]r,aq is the remaining Cu(II) concentration in the aqueous phase after extraction.
Theory
The extraction process may be represented by the equation,
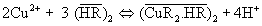 (2)
(2)
where 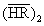 represents the extraction reagent. The extraction constant of the species
represents the extraction reagent. The extraction constant of the species 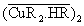 is given by:
is given by:
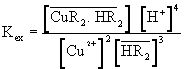 (3)
(3)
where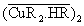 is the only extractable species. Introducing the mass balance equation for the Cu(II)and the extractant
is the only extractable species. Introducing the mass balance equation for the Cu(II)and the extractant
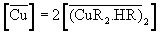 (4)
(4)
The mass balance equation for the Cu(II) in the aqueous phase
[Cu]= [Cu2+] + [CuR+] + [CuR2] + [CuSO4] (5)
[Cu]= 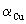 [Cu2+](6)
[Cu2+](6)
where 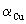 is formation constant of Cu(II). The extraction constant of the total Copper in the aqueous phase is given by
is formation constant of Cu(II). The extraction constant of the total Copper in the aqueous phase is given by
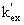 =
= 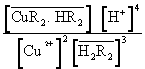 (7)
(7)
The metal distribution ratio (D’) and the extraction constant are related by
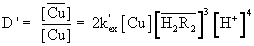 (8)
(8)
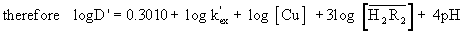 (9)
(9)
According to (Equation 9) a plot of logD’ versus pH will give straight line shown in Figures 1, 2. The distribution coefficients (D’) increase with increasing pH. A plot of log D’ – log [Cu] against pH (Figure 3) will give a straight line of slope and intercept log Kex at constant concentration of log 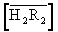 in Table 1. The Figure 4 log D’ –log [Cu] - 4pH versus of log
in Table 1. The Figure 4 log D’ –log [Cu] - 4pH versus of log 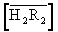 will show a straight line of slope and intercept log Kex. Parameter correlation equilibrium data for extraction of Cu(II) using lauric acid diluted in benzene at 298.2 K presented in Table 2. The extraction constant (log Kex) has been calculated as -12.2580.
will show a straight line of slope and intercept log Kex. Parameter correlation equilibrium data for extraction of Cu(II) using lauric acid diluted in benzene at 298.2 K presented in Table 2. The extraction constant (log Kex) has been calculated as -12.2580.
Figure 3: The plots of log D’ –log [Cu] versus pH at the extraction of Cu(I) with lauric acid diluted in benzene at 298.2 K. Initial concentration of Cu(I) (M): concentration of 0.2 M lauric acid: (●) 5×10-4; (○) 2.5×10-3; (Δ) 5×10-3; (▲) 2.5×10-2 and concentration of 0.5 M lauric acid: (■) 5×10-4; (□) 2.5×10-3; (×) 5×10-3; (◊) 2.5×10-2 .
 ×10-3 ×10-3(mol-g/dm3) |
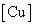 ×10-3 ×10-3(mol-g/dm3) |
D' | pH | 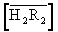 (mol-g/dm3) |
| 1.88 | 3.12 | 0.60 | 4.52 | 0.05 |
| 1.89 | 3.11 | 0.61 | 4.30 | 0.10 |
| 1.91 | 3.09 | 0.62 | 4.18 | 0.15 |
| 1.99 | 3.01 | 0.66 | 4.05 | 0.25 |
| 2.05 | 2.95 | 0.70 | 3.91 | 0.35 |
| 2.09 | 2.91 | 0.72 | 3.80 | 0.50 |
Table 1: Equilibrium data of (CuSO4 + lauric acid + benzene) at 298.2 K: influence of luaric acid concentration. Ccu,initial = 5×10-3 M , CNa2SO4 = 0.1 M (14.204 g/dm3).
| C cu,initial (mol-g/dm3) |
Log D'- log [Cu] versus pH | ||||||
| C acid=0.2 M | C acid=0.5 M | ||||||
| a | b | R2 | a | b | R2 | ||
| 5.0×10-4 | 3.9884 | -15.460 | 0.9984 | 3.971 | -13.907 | 0.9997 | |
| 2.5×10-3 | 3.5625 | -13.412 | 0.9294 | 4.0632 | -14.070 | 0.9999 | |
| 5.0 ×10-3 | 3.9939 | -14.976 | 0.9996 | 3.5488 | -11.741 | 0.9906 | |
| 2.5×10-2 | 4.0035 | -15.180 | 0.9907 | 3.9927 | -13.227 | 0.9999 | |
Table 2: Parameter correlation equilibrium data for extraction of Cu(II) with using lauric acid diluted in benzene at 298.2 K.
Data extraction equilibrium for initial Cu (II) concentration (5 X 10-4, 2.5 X 10-3, 5X 10-3 and 2.5 X 10-2 ) M in pH=1.6 Sulphuric acid solution in the range of 0.2 - 0.5 M Lauric acid, pH for extraction, distribution coefficient (D’), percentage extraction (%E) given in Tables 3-6 at atmospheric pressure and at T=298.2 K. Table 1 gives influence of lauric acid concentration of the equilibrium data of CuSO4 and lauric acid diluted in benzene.
| Cacid = 0.2 M (40.07 g/dm3) | Cacid = 0.5 M (100.17 g/dm3) | ||||||||||
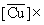 10-4 10-4(mol-g /dm3 ) |
[Cu]×10-4 (mol-g /dm3) |
D’ | %E | pH | 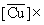 10-4 10-4( mol-g /dm3 ) |
[Cu]×10-3 (mol-g /dm3) |
D’ | %E | pH | ||
| 0.866 | 4.14 | 0.21 | 17.3 | 4.56 | 0.775 | 4.23 | 0.18 | 15.5 | 4.17 | ||
| 1.15 | 3.86 | 0.30 | 22.9 | 4.59 | 1.07 | 3.93 | 0.27 | 21.5 | 4.22 | ||
| 1.58 | 3.42 | 0.46 | 31.6 | 4.67 | 1.70 | 3.30 | 0.52 | 34.1 | 4.30 | ||
| 2.01 | 2.99 | 0.67 | 40.1 | 4.70 | 1.98 | 3.06 | 0.65 | 39.6 | 4.34 | ||
| 2.21 | 2.80 | 0.79 | 44.1 | 4.74 | 2.55 | 2.45 | 1.04 | 51.0 | 4.42 | ||
| 2.27 | 2.73 | 0.83 | 45.4 | 4.76 | 3.87 | 1.13 | 3.43 | 77.4 | 4.63 | ||
| 2.96 | 2.04 | 1.46 | 59.3 | 4.86 | 4.24 | 0.759 | 5.59 | 84.8 | 4.72 | ||
| 3.72 | 1.28 | 2.90 | 74.4 | 4.96 | 4.62 | 0.386 | 12.0 | 92.3 | 4.88 | ||
| 4.03 | 0.975 | 4.13 | 80.5 | 5.03 | 4.72 | 0.284 | 16.6 | 94.3 | 4.95 | ||
| 4.86 | 0.147 | 33.1 | 97.1 | 5.47 | 4.88 | 0.119 | 40.9 | 97.6 | 5.16 | ||
Table 3: Equilibrium data of (CuSO4 + lauric acid + benzene) at 298.2 K. Ccu,initial = 5×10-4 M (0.0318 g/dm3) , CNa2SO4 = 0.1 M (14.204 g/dm3).
| Cacid = 0.2 M (40.07 g/dm3) | Cacid = 0.5 M (100.17 g/dm3) | ||||||||
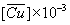 (mol-g /dm3 ) |
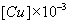 ( mol-g /dm3 ) |
D' | %E | pH | 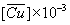 (mol-g /dm3 ) |
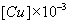 ( mol-g /dm3 ) |
D' | %E | pH |
| 0.310 | 2.19 | 0.14 | 12.4 | 4.26 | 0.171 | 2.33 | 0.07 | 6.8 | 3.83 |
| 0.454 | 2.05 | 0.22 | 18.2 | 4.31 | 0.419 | 2.08 | 0.20 | 16.8 | 3.95 |
| 0.638 | 1.86 | 0.34 | 25.5 | 4.36 | 0.790 | 1.71 | 0.32 | 31.6 | 4.02 |
| 0.691 | 1.81 | 0.38 | 27.6 | 4.39 | 0.945 | 1.56 | 0.61 | 37.8 | 4.10 |
| 0.974 | 1.53 | 0.64 | 39.0 | 4.46 | 0.122 | 1.28 | 0.96 | 48.9 | 4.17 |
| 1.03 | 1.47 | 0.89 | 41.2 | 4.50 | 0.172 | 0.784 | 2.19 | 68.7 | 4.31 |
| 1.40 | 1.10 | 1.28 | 56.1 | 4.56 | 0.191 | 0.587 | 3.26 | 76.5 | 4.38 |
| 1.85 | 6.52 | 2.83 | 73.9 | 4.71 | 2.05 | 0.447 | 4.60 | 82.1 | 4.45 |
| 2.25 | 2.52 | 8.91 | 89.9 | 4.94 | 2.09 | 0.412 | 5.07 | 83.5 | 4.47 |
| 2.48 | 0.02 | 123 | 99.2 | 5.54 | 2.15 | 0.353 | 6.08 | 85.9 | 4.51 |
Table 4: Equilibrium data of (CuSO4 + lauric acid + benzene) at 298.2 K. Ccu,initial = 2.5×10-3 M (0.1589 g/dm3) , CNa2SO4 = 0.1 M (14.204 g/dm3).
| Cacid = 0.2 M (40.07 g/dm3) | Cacid = 0.5 M (100.17 g/dm3) | |||||||||
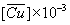 (mol /dm3 ) (mol /dm3 ) |
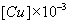 ( mol /dm3 ) |
D' | %E | pH | 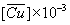 (mol /dm3 ) (mol /dm3 ) |
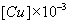 ( mol /dm3 ) |
D' | %E | pH | |
| 0.309 | 4.69 | 0.07 | 6.2 | 4.05 | 0.148 | 4.85 | 0.03 | 3.0 | 3.57 | |
| 0.422 | 4.58 | 0.09 | 8.4 | 4.08 | 0.293 | 4.71 | 0.06 | 5.9 | 3.65 | |
| 0.603 | 4.40 | 0.14 | 12.1 | 4.12 | 0.463 | 4.54 | 0.10 | 9.3 | 3.71 | |
| 1.35 | 3.65 | 0.37 | 27.0 | 4.25 | 0.536 | 4.47 | 0.13 | 11.3 | 3.74 | |
| 2.35 | 2.66 | 0.88 | 47.0 | 4.38 | 1.47 | 3.53 | 0.42 | 29.4 | 3.89 | |
| 2.40 | 2.60 | 0.92 | 48.0 | 4.39 | 1.89 | 3.11 | 0.61 | 37.8 | 3.94 | |
| 2.49 | 2.51 | 0.99 | 49.8 | 4.40 | 2.10 | 2.91 | 0.72 | 42.0 | 3.97 | |
| 3.12 | 1.88 | 1.66 | 62.4 | 4.48 | 2.55 | 2.46 | 1.04 | 51.2 | 4.03 | |
| 3.34 | 1.66 | 2.04 | 67.2 | 4.52 | 3.15 | 1.85 | 1.70 | 63.0 | 4.12 | |
| 3.64 | 1.36 | 2.68 | 72.8 | 4.57 | 3.80 | 1.21 | 3.14 | 76.0 | 4.22 | |
| 4.24 | 0.762 | 5.56 | 84.8 | 4.72 | 4.83 | 1.69 | 25.6 | 96.7 | 4.68 | |
| 4.63 | 0.370 | 12.5 | 92.6 | 4.89 | 4.99 | 1.22 | 396 | 99.7 | 5.24 | |
Table 5: Equilibrium data of (CuSO4 + lauric acid + benzene) at 298.2 K. Ccu,initial = 5×10-3 M (0.3177 g/dm3) , CNa2SO4 = 0.1 M (14.204 g/dm3).
| Cacid = 0.2 M(40.07 g/dm3) | Cacid = 0.5 M(100.17 g/dm3) | |||||||||
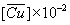 (mol /dm3 ) (mol /dm3 ) |
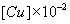 ( mol /dm3 ) |
D' | %E | pH | 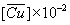 (mol /dm3 ) (mol /dm3 ) |
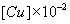 (mol /dm3 ) (mol /dm3 ) |
D' | %E | pH | |
| 0.067 | 2.43 | 0.03 | 2.7 | 3.86 | 0.205 | 2.23 | 0.09 | 8.2 | 3.46 | |
| 0.10 | 2.40 | 0.04 | 4.0 | 3.88 | 0.393 | 2.11 | 0.19 | 15.7 | 3.55 | |
| 0.132 | 2.37 | 0.06 | 5.3 | 3.89 | 0.467 | 2.03 | 0.23 | 18.7 | 3.58 | |
| 0.136 | 2.36 | 0.06 | 5.4 | 3.90 | 0.629 | 1.87 | 0.34 | 25.2 | 3.63 | |
| 0.150 | 2.35 | 0.06 | 6.0 | 3.91 | 0.735 | 1.77 | 0.42 | 29.4 | 3.66 | |
| 0.199 | 2.30 | 0.09 | 8.0 | 3.93 | 0.889 | 1.61 | 0.55 | 35.5 | 3.70 | |
| 0.216 | 2.28 | 0.09 | 8.6 | 3.96 | 1.20 | 1.30 | 0.92 | 48.0 | 3.78 | |
| 0.381 | 2.12 | 0.18 | 15.2 | 3.98 | 1.51 | 0.996 | 1.51 | 60.2 | 3.86 | |
| 0.724 | 1.78 | 0.41 | 29.0 | 4.09 | 1.74 | 0.763 | 2.28 | 69.5 | 3.93 | |
| 0.934 | 1.57 | 0.60 | 37.4 | 4.15 | 1.82 | 0.676 | 2.70 | 73.0 | 3.96 | |
| 1.10 | 1.41 | 0.78 | 43.8 | 4.20 | 1.95 | 0.551 | 3.54 | 78.0 | 4.02 | |
| 1.41 | 1.09 | 1.29 | 56.4 | 4.31 | 2.01 | 0.493 | 4.08 | 80.4 | 4.04 | |
| 1.59 | 0.908 | 1.80 | 63.7 | 4.36 | 2.10 | 0.401 | 5.24 | 84.0 | 4.09 | |
| 1.69 | 0.807 | 2.10 | 67.6 | 4.40 | 2.23 | 0.274 | 8.12 | 89.0 | 4.18 | |
| 1.89 | 0.614 | 3.07 | 75.4 | 4.49 | 2.47 | 0.033 | 73.9 | 98.7 | 4.65 | |
| 2.35 | 0.152 | 15.4 | 93.9 | 4.81 | 0.25 | 0.003 | 874 | 99.5 | 5.19 | |
Table 6: Equilibrium data of (CuSO4 + lauric acid + benzene) at 298.2 K. Ccu,initial = 2.5×10-2 M (1.5886 g/dm3) , CNa2SO4 = 0.1 M (14.204 g/dm3).
Effect of pH and Extractant concentration
Creature Cu (II) extraction by lauric acid depend on the initial acidity of the aqueous solution, more studied were carried out in order to recognize the influence of the aqueous pH on Cu (II) extraction. The pH will be very essential factor in the separation of metal ion. The results obtained were shown in Figs. 5 and 6, plotting the percentage of copper extraction (%E) against equilibrium pH. From Figures 5 and 6 find the lowest at pH of 3.46 and maximum pH of 5.54. The extraction percentage (%E) increased with growth of pH. It also can be seen that the curves are shifted to the left as the increase of initial Cu (II) concentration.
Thermodynamic part
Free energy  (thermodynamic parameter) concerned in this study due to the transfer of a unit mole of Cu (II) from the aqueous into the organic phases. The
(thermodynamic parameter) concerned in this study due to the transfer of a unit mole of Cu (II) from the aqueous into the organic phases. The  of Cu (II) extraction at T=298.2 K was determined from [13]
of Cu (II) extraction at T=298.2 K was determined from [13]
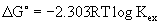 (10)
(10)
Where R is the universal gas constant (8.314 J/mol K), T is the thermodynamic temperature and Kex is the extraction constant and this was presented in Table 7 and the  value is negative. The negative of
value is negative. The negative of  indicated that the Cu (II) extraction with lauric acid occurred spontaneously at T=298.2 K. From log Kex versus log [Cu] (Figure 7) the extraction constant (log Kex increased with increasing Cu (II) concentration in aqueous phase.
indicated that the Cu (II) extraction with lauric acid occurred spontaneously at T=298.2 K. From log Kex versus log [Cu] (Figure 7) the extraction constant (log Kex increased with increasing Cu (II) concentration in aqueous phase.
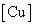 10-3 10-3(mol-g/dm3) |
Kex | HR (kJ/mol) |
| 3.12 | 9.248×10+23 | -136.838 |
| 3.11 | 1.554×10+22 | -126.707 |
| 3.09 | 1.560×10+21 | -121.006 |
| 3.01 | 1.112×10+20 | -114.457 |
| 2.95 | 1.208×10+19 | -108.952 |
| 2.91 | 1.568×10+18 | -103.890 |
Table 7: The extraction constant and free energy  of equilibrium data for extraction of Cu (II) with using lauric acid diluted in benzene at 298.2 K.
of equilibrium data for extraction of Cu (II) with using lauric acid diluted in benzene at 298.2 K.
The experimental data indicate lauric acid in organic phase extracts capably Cu(II) from aqueous solutions in the pH =1.6 Sulphuric acid at T=298.2 K under atmospheric pressure. The distribution coefficient (D’) and extraction percentage (%E) increased with growth of pH. The results indicate pH, percentage extraction (%E), and distribution coefficient (D’) decrease with increasing initial Cu(II) concentration (5 X 10-4 to 2.5 X 10-2) M and they raise with increasing lauric acid concentration 0.2 to 0.5 M. The negative of 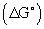 indicated that the Cu(II) extraction with lauric acid occurred spontaneously.
indicated that the Cu(II) extraction with lauric acid occurred spontaneously.