Journal of Oceanography and Marine Research
Open Access
ISSN: 2572-3103
ISSN: 2572-3103
Research Article - (2021)Volume 9, Issue 6
Introduction: Acute Pancreatitis (AP) is an inflammatory condition having varied presentation ranging from a mild self-limiting illness to a severe disease with multi organ failure. Excessive recruitment of leukocytosis is an important pathophysiological feature and Myelo-Peroxidase (MPO) forms an important part of neutrophil induced inflammation. The current prognostic criteria are complex and cumbersome.
Materials and methods: The present cross sectional pilot study serial estimation of plasma MPO levels were done at the time of admission and on 3, 7 and 14 days in patient of Acute Pancreatitis (n=64) patient with acute abdominal symptoms (n=15) otherthan acute pancreatitidand healthy volunteers (n=15). The values of serum MPO levels were correlated with Ransons`s score, Apache score, CT severity index and patient developing local and systemic complications due to acute pancreatitis. Serum MPO levels in (mU/ml) were measured by colorimetric Assay kit (bio Vision, USA). Statistical Analysis on SPSS (Windows version 21.0)
Results: The mean serum MPO levels were significantly high in patients of AP as compared to controls comprising of patients with acute for to abdominal conditions and healthy individuals (mean 12.73 vs. 1.67 mU/ml. p<0.001). The highest levels of serum MPO were observed on the first day of mild and severe AP. The mean MPO levels in mild diseases (n=21 patients) were 3-10 mU/ml and 10-20 mU/ml in severe AP (n=43 patients) who also experienced higher local complications and worse outcome in terms of mortality. MPO returned to normal levels within 7-10days in mild but were persistently raised in patients with severe diseases.
Conclusion: The higher serum MPO levels correlated with more severe diseases, worse outcome and can be a simple and effective prognostic indicator in AP.
Severe pain; Biochemical marker; Pancreatitis
Acute pancreatitis is a common clinical condition, the incidence of which has been increasing over recent years. It is an inflammatory condition characterized by a wide clinical spectrum ranging from a mild self-limiting to a severe, systemic disease. Majority of patients experience a mild form, but up to 20 percent develop necrotizing acute pancreatitis associated with a mortality rate of 10-30 percent.
The rationale for assessing the severity of acute pancreatitis is mainly practical: mild pancreatitis responds well to supportive therapy, whereas severe pancreatitis requires intensive monitoring and specific therapies and has a more guarded prognosis. It is, therefore, essential to identify the subgroup of patients which will go on to develop adverse outcomes and severe disease, early on in the disease course. This is where accurate predictors of severity and adverse outcome in acute pancreatitis would find a place [1].
An ideal predictor that allows differentiation between patients with mild and those with severe pancreatitis should be simple, inexpensive, accurate, reproducible, and widely available, contain few parameters and should have low inter-observer variability. It should also be applicable early in the disease process, so that patients who could potentially develop complications can be monitored more closely or empirically treated, for example with fluid resuscitation.
Different strategies have been used to assess the severity of acute pancreatitis and predict outcome. Several clinical scoring systems (Ranson’s criteria, Glasgow, Imrie) are available. The APACHE II scoring system, though cumbersome due to consideration of multiple parameters, appears to be the best validated. Biological markers have also been used for this purpose. Genetic markers are being studied and have not yet come into clinical use. Dynamic CT scanning of the abdomen is widely available and useful in predicting the outcome of acute pancreatitis by CT-based scoring systems [2].
Even though a number of single and multi-parameter severity predictors of acute pancreatitis have been described and studied over the past several years, most of them are still far from perfect.
An important characteristic feature of acute pancreatitis is the pancreatic inflammation with excessive recruitment of leukocytes. Inflammatory mediators appear to play a critical role in the pathogenesis of pancreatitis and more so of the subsequent inflammatory response. Enzymes contained in azurophilic granules (acid and neutral proteinases, acid phosphatase, and myeloperoxidase) are responsible for delayed type phase of bacteriolysis. Derivatives of myeloperoxidase oxidation can evoke the liberation of vasoactive amines from platelets and mast cells. Increased myeloperoxidase blood levels reflect neutrophil activation. Myeloperoxidase has been associated with respiratory complications associated with severe acute pancreatitis. However, myeloperoxidase blood levels have not been yet studied for diagnosis of acute pancreatitis and predicting the future disease course and prognosis in these patients [3].
Through our study, we try to formulate serum Myeloperoxidase (MPO) as a diagnostic as well as a prognostic marker for patients of acute pancreatitis. The aim of our study was to grade the severity status of pancreatitis on the basis of raised MPO levels and to assess if there is any correlation of MPO levels with the known existing prognostic markers and clinical outcome of the patients of acute pancreatitis.
Objectives
To grade the severity status of pancreatitis on the basis of raised MPO levels.
To assess the correlation of MPO levels with the known existing prognostic markers.
To correlate the outcome of the patients of acute pancreatitis with the raised MPO levels
The present cross-sectional pilot study was conducted in the department of General Surgery, King George’s Medical University, and Lucknow; in collaboration with the department of Pathology, King George’s Medical University, Lucknow from August, 2012 to August, 2013 and Ethical clearance was obtained from the institutional ethical committee. The study subjects were the patients of acute pancreatitis attending the emergency surgical ward/OPD of department of General Surgery, King George’s Medical University, Lucknow. The diagnosis of acute pancreatitis was made on the basis of clinical symptoms, associated with a serum amylase and lipase concentration 3 times more than normal; supported by ultrasonographic and/or CT scan findings. The serial levels of MPO were correlated with the current prognostic criteria of pancreatitis (Ranson’s Prognostic Criteria, Computed Tomography Severity Index or CTSI, Acute Physiology and Chronic Health Evaluation Scoring or APACHE) [4].
The control samples were taken from patients presenting with acute abdominal pain due to causes (Acute appendicitis, Acute Cholecystitis, Acute Intestinal Obstruction, Perforation Peritonitis, Carcinoma Gall Bladder, Obstructed Inguinal hernia) other than acute pancreatitis and healthy individuals. The exact reason why MPO levels should be different in two acute inflammatory conditions is still not clear however an intense inflammatory reaction and some unknown inflammatory pathway might be the reason for earliest differences in MPO levels in both the groups. All the patients of acute pancreatitis were categorized on the basis of Ranson’s prognostic criteria; differently for gallstone-induced and nongallstone-induced pancreatitis; into two groups i.e. mild and severe. The cases were categorized into mild and severe pancreatitis based on APACHE scoring system.
Severity of acute pancreatitis was assessed by taking into consideration the presence of local complications and/or presence of organ failure whether transient or persistent. The organ failure was labeled as transient if it resolves within 48 hours.
Inclusion criteria
Cases and controls.
Patients diagnosed of acute pancreatitis and presenting within 24 hours of onset of pain.
Patients having abdominal pain due to causes other than acute pancreatitis (on the basis of clinical findings and radiological investigations viz. CT abdomen).
Normal healthy adults/volunteers.
Exclusion criteria
Patients diagnosed of acute pancreatitis presenting late to hospital (>24hours of onset of pain).
Patients having chronic diseases like malignancy and chronic infectious disorders.
Patients who left against medical advice from the surgical wards.
Since it was a cross-sectional pilot study, no formal sample size was calculated. However, we included 64 subjects. The venous blood samples (5 mL) of patients; cases as well as controls; were taken immediately on admission, and on days 3, 7, and 14 after admission. Serum it was separated by centrifugation at 3000 rpm for 10 minutes at room temperature. Serum samples were stored in eppendorf vials in deep freezer at a temperature of 70 degree Celsius till analysis. Sample analysis was done with Myeloperoxidase (MPO) Activity Colorimetric Assay Kit (BioVision, USA) as mentioned in previous studies. The patients were followed throughout their hospital stay up to time of discharge from the hospital. The patients were categorized as improved or expired [5].
Statistical analysis
Data was summarized as Mean ± SD. Two independent groups were compared by independent Student’s test. Two dependent groups were compared by Wilcoxon (W) matched pairs test and more than two groups were compared with ANOVA. Discrete (categorical) groups were compared by chi-square test. All analyses were performed on SPSS (Windows version 21.0) [6].
A total of 94 patients were enrolled in this study. 64 patients of acute pancreatitis fulfilling the inclusion criteria were recruited and evaluated which served as “cases”. 30 individuals comprising of volunteers/healthy adults (n=15) and patients having abdominal pain due to causes (n=15) other than acute pancreatitis were also recruited and served as “controls”.
Basic Characteristics
The mean age of cases and controls was 43.016 ± 13.7673 years and 43.133 ± 11.5661 years respectively, and median of 45 in both (43.133 ± 11.5661 vs. 43.016 ± 13.7673; p=0.238).
There were 37 (57.8%) males and 27 (12.2%) females in cases and 16 (53.5%) males and 14 (46.7%) females in the control group respectively.
Out of all the cases studied the maximum patients had gallstone induced (n=24, 37.5%) and alcohol induced pancreatitis (n=18, 28.1%). The subjects in control group comprised of healthy adults and patients of acute abdomen other than pancreatitis [7].
Levels of pancreatic enzymes i.e. amylase and lipase, were found to be significantly raised in patients of acute pancreatitis with mean values of 1160.0 and 1137.3 respectively.
Majority of patients were having severe pancreatitis (87.5%) according to Ranson’s prognostic criteria. The majority of patients had severe pancreatitis (67.2%) according to APACHE classification. The patients of acute pancreatitis were grouped according to their CTSI values with maximum patients having CTSI of 6 (32.8%) followed by CTSI of 8 and 4.
Presence of complications in patients of acute pancreatitis
The patients of acute pancreatitis were further grouped depending on presence of local complications. Most patients either had no local complications or developed acute peripancreatic sterile fluid collection (both 37.5%) [8].
The patients of acute pancreatitis were classified into four categories taking into consideration presence of local complications and organ failure. About half of the patients had moderate severity (50.0%) i.e. they had sterile (peri) pancreatic complication or transient organ failure. The final outcome was also studied in all the patients of acute pancreatitis and majority of patients improved during their stay in the hospital (82.8%).
Myeloperoxidase levels in cases and controls
The mean MPO levels of cases were observed to be significantly higher than mean MPO levels of controls on admission and days 3, 7 and 14. The mean MPO levels of cases on admission were observed to be highest as compared to mean MPO levels on day 3, 7 and 14 and the difference was statistically significant (p=0.03). Whereas the mean MPO levels were found to be almost similar in controls on all different occasions. The difference in mean MPO levels in cases and controls on admission was statistically significant Table 1.
| Group | MPO levels on admission | MPO levels on day 3 | MPO levels on day 7 | MPO levels on day 14 | P value |
|---|---|---|---|---|---|
| Cases (n=64) Mean ± SD | 12.73591 ± 4.363754 |
11.24641 ± 4.250051 |
8.398547 ± 3.8688375 | 6.68592 ± 4.2 59717 |
<0.05 |
| Controls(n=30) Mean ± SD |
1.67007 ± 807782 | 1.45240 ± 0.807782 |
1.493470 ± 0.807782 |
1.38813 ± 0.807782 |
Table 1: Mean MPO levels (mU/mL) in cases and controls on different days.
Myeloperoxidase levels in relation to ranson severity
The mean MPO levels in cases of mild and severe pancreatitis were 7.05 and 13.55 respectively. The difference was statistically significant (p<0.05). The mean MPO levels of both the groups decreased over time duration. The higher MPO levels at the time of admission correlated with severe disease according to Ranson’s prognostic index (mean 6.85) Table 2.
| Ranson’s Severity | MPO levels on admission | MPO levels on day 3 | MPO levels on day 7 | MPO levels on day 14 | P value |
|---|---|---|---|---|---|
| Mild (n=8) Mean ± SD |
7.05 ± 2.032 | 6.16 ± 2.366 | 4.28 ± 2.233 | 2.49 ± 0.911 | <0.05 |
| Severe(n=56) Mean ± SD |
13.55 ± 3.990 | 11.97 ± 3.958 | 8.99 ± 3.700 | 7.29 ± 4.213 |
Table 2: Mean MPO levels (mU/mL) in groups according to Ranson’s score (p<0.05).
Myeloperoxidase levels in relation to apache score
The patients labeled as having severe pancreatitis according to APACHE had higher mean MPO levels on all occasions in comparison to those labeled as having mild pancreatitis. The means of both the groups were compared and the difference was found to be statistically significant (p<0.05). With more severe disease according to APACHE scoring the myeloperoxidase levels were also observed to be higher. The higher mean MPO levels at admission correlated with more severe disease according to APACHE (mean 8.2) Table 3.
| APACHE Severity | MPO levels on admission | MPO levels on day 3 | MPO levels on day 7 | MPO levels on day 14 | P value |
|---|---|---|---|---|---|
| Mild (n=21) Mean ± SD |
7.97 ± 2.269 | 6.63 ± 2.162 | 4.34 ± 1.733 | 2.96 ± 1.463 | <0.05 |
| Severe (n=43) Mean ± SD |
15.06 ± 3.040 | 13.50 ± 3.005 | 10.38 ± 2.962 | 8.50 ± 3.985 |
Table 3: Mean MPO levels (mU/mL) in relation to APACHE severity (p<0.05).
Myeloperoxidase levels in relation to CTSI
The higher mean MPO levels were seen in patients having more CTSI. The patients having CTSI of 10 had mean MPO levels of 18.58 ± 2.454 and patients having CTSI of 4 had mean MPO levels of 8.83 ± 2.823. The mean MPO levels at admission were compared among groups having CTSI 4,6,5,8,10 and differences were found to be significant by one way ANOVA test (p value <0.05) with one exception i.e. the levels of MPO were not statistically different among groups with CTSI 8 and 10 on all the four occasions. The levels of serum MPO were observed to increase as the CT severity index increases. The patients with lower CTSI had lower mean serum MPO levels Table 4.
| CT severity index | MPO levels on dmission | MPO levels on day 3 | MPO levels on day 7 | MPO levels on day 14 | P value |
|---|---|---|---|---|---|
| 4(n=19) Mean ±SD |
8.83 ± 2.823 | 7.15 ± 2.337 | 4.97 ± 2.218 | 3.23 ± 1.731 | < 0.05 |
| 5 (n=1) Mean ±SD |
7.30 | 5.24 | 4.45 | 4.02 | |
| 6 (n=21) Mean ±SD |
12.33 ± 2.743 | 10.71 ± 2.676 | 7.89 ± 2.746 | 5.79 ± 2.177 | |
| 8 (n=19) Mean ±SD |
16.14 ± 3.402 | 15.26 ± 2.649 | 12.02 ± 2.919 | 10.56 ± 3.933 | |
| 10 (n=04) Mean ±SD |
18.58 ± 2.454 | 15.93 ± 2.311 | 11.17 ± 2.918 | 10.03 ± 6.713 |
Table 4: Mean MPO levels (mU/mL) in patients with different CTSI (p value <0.05).
Myeloperoxidase levels in relation to clinical classification
The serum MPO levels of patients stratified into different groups according to clinical classification system were studied. The highest levels were seen in patients in critical group with mean ± SD MPO levels of 19.18 ± 1.942 mU/mL at admission (Figure 1).
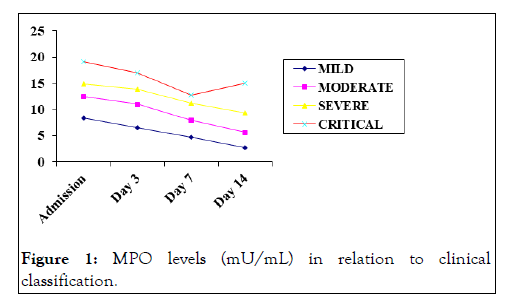
Figure 1: MPO levels (mU/mL) in relation to clinical classification.
The lowest levels of MPO were seen in mild group with mean ± SD of 8.40 ± 2.257. The independent variables of MPO on different occasions were tested by one way ANOVA. The difference between means was statistically significant on three occasions i.e. on admission, day 3 and day 14 (p<0.05). The difference between mean MPO values was not statistically significant on day 7 between patients having severe and critical disease (p=0.314); the difference was significant between rest of the groups (p<0.05) [9].
Myeloperoxidase levels and organ failure
The highest MPO levels at admission were observed in patients having persistent organ failure with mean ± SD of 17.03 ± 3.19 mU/mL. Patients having no organ failure had lowest mean MPO levels of 3.82 ± 1.833mU/mL on day 14. The higher levels of MPO correlated with higher levels of organ failure. The mean values of serum MPO decrease with the disease course. But when paired t-test was applied the patients having persistent organ failure had statistically insignificant differences between the MPO levels on admission and day 14 (p=0.52) [10].
Myeloperoxidase levels and local complications
The maximum mean MPO levels in relation to presence of local complications was observed in patients having infected pancreatic necrosis with mean ± SD of 19.47 ± 2.32 mU/mL and the difference was clinically significant when compared to other groups. Patients who had no local complications had the lowest values with mean ± SD of 9.43 ± 3.03 mU/mL The mean value of MPO at admission in patients having no complications was lower when compared to mean MPO levels of patients having various complications and was statistically significant (p<0.05). Even though the mean values of patients developing pancreatic pseudocyst, acute sterile peripancreatic fluid collection and acute infected peripancreatic fluid collection were different but this difference was statistically insignificant (p=0.6) (Table 5 and Figure 2) [11].
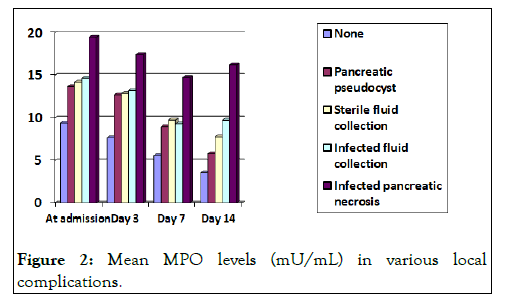
Figure 2: Mean MPO levels (mU/mL) in various local complications.
| Local Complications | MPO levels on dmission | MPO levels on day 3 | MPO levels on day 7 | MPO levels on day 14 | P value |
|---|---|---|---|---|---|
| None (n=24) Mean ± S.D |
9.43525 ± 3.030792 | 7.71033 ± 2.738064 | 5.58254 ± 2.896832 | 3.57475 ± 1.970926 | < 0.05 |
| Pancreatic pseudocyst (n=7) Mean ± S.D |
13.69357 ± 2.642548 | 12.72929 ± 1.506899 | 9.01100 ± 1.881638 | 5.81143 ± 1.712109 | |
| Acute peripancreatic sterile fluid collection (n=24) Mean ± S.D |
14.22596 ± 3.468671 | 12.90067 ± 3.580319 | 9.77313 ± 3.341285 | 7.81513 ± 3.155459 | |
| Acute peripancreatic infected fluid collection (n=5) Mean ± S.D |
14.69680 ± 5.736667 | 13.23320 ± 4.708549 | 9.35040 ± 3.716209 | 9.75720 ± 4.370355 | |
| Infected pancreatic necrosis (n=4) Mean ± S.D |
19.47250 ± 2.324537 | 17.45875 ± 2.091768 | 14.78550 ± 1.890064 | 16.26900 ± 4.001614 |
Table 5: Mean MPO levels (mU/mL) in relation to local complications.
Myeloperoxidase levels in relation to outcome
The mean serum myeloperoxidase levels were higher at all the occasions in patients who expired. The mean MPO levels were persistently raised in patients who expired with statistically insignificant difference between MPO levels on days 7 and 14 (p>0.05). The MPO levels in those who improved showed significant difference in mean values on days 3, 7 and 14 (p<0.05).The myeloperoxidase levels among controls were observed to be lower in patients having chronic conditions and healthy adults (Table 6 and Figure 3).
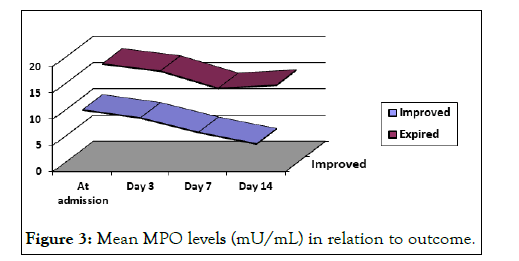
Figure 3: Mean MPO levels (mU/mL) in relation to outcome.
| Outcome | MPO levels on admission | MPO levels on day 3 | MPO levels on day 7 | MPO levels on day 14 | P value |
|---|---|---|---|---|---|
| Improved (n=53) Mean ± S.D |
11.73040 ± 3.964664 | 10.21272 ± 3.792214 |
7.45915 ± 3.407433 |
5.26470 ± 2.704131 |
> 0.05 |
| Expired (n=11) Mean ± S.D |
17.58064 ± 2.655786 | 16.22691 ± 2.463085 |
12.92473 ± 2.590494 |
13.53364 ± 3.742315 |
Table 6: Mean MPO levels (mU/mL) in relation to outcome.
Taken together, the present study suggests that serum MPO levels are raised in patients of acute pancreatitis at day of admission as compared to normal healthy adults and patients suffering from other similar acute abdominal conditions. The serum MPO levels in patients of acute pancreatitis were observed to fall over time course signifying maximal inflammatory response at disease onset. The comparisons carried out between serum MPO levels and known prognostic indicators like Ranson’s score, APACHE and CT severity index clearly showed the higher serum MPO levels to correlate with patients labelled as having severe disease by these indicators [12].
The metabolic alterations that occur in patients of acute pancreatitis are mediated by polymorphonuclear leukocytes. An early event in pathogenesis of acute pancreatitis is local inflammation of the pancreas followed by generalised inflammatory response. A major role in polymorphonuclear dependent microbicidal activity is played by Myeloperoxidase- H2O2-Halide system. Myeloperoxidase is a heme enzyme released by activated neutrophils, from intracellular granules. Myeloperoxidase levels in serum have been observed to be increased in various disease states like acute respiratory distress syndrome, sepsis and septic shock, bronchial asthma, juvenile arthritis etc. In studies on animal models of pancreatitis that higher levels of tissue myeloperoxidase correlated with increased pulmonary microvascular permeability in caerulein-induced pancreatitis. The increased myeloperoxidase activity was shown to be associated with pancreatitis associated lung injury. In a study carried out on Human leukocytic cell line HL-60 cells almost similar results of excessive levels of MPO was reported by in patients of coronary artery diseases and selective blockage of signaling pathway by niacin , suppressed neutrophil MPO release responsible for coronary heart diseases [13].
In a large study of 237 patients of sepsis, concluded from their study that higher levels of serum myeloperoxidase correlated with increased disease severity and sepsis related complications. Similarly, studied 86 patients with acute pancreatitis (48 patients with mild and 38 patients with severe pancreatitis). The relations of serum myeloperoxidase levels to cytokine level, severity, and time-course of pancreatitis were studied. Their study showed the clear dependence of MPO blood level on the severity of acute pancreatitis and on cytokine blood levels. Their study also provided an insight in the therapeutic role of Pentoxifylline in cases of severe acute pancreatitits as histologic lung injury, pulmonary neutrophil activity and proinflammatory signaling was attenuated after therapy with this novel agent.
Similar results have been found in our study as serum myeloperoxidase levels were higher in patients of acute pancreatitis on day of admission when compared to healthy adults and patients with other disease states. This difference was statistically significant (p<0.05). This relates to the amount of local and systemic inflammatory response seen in patients of acute pancreatitis. The serum myeloperoxidase levels were observed to fall to significant levels as the time elapsed after onset of acute pancreatitis. This fall can be partly explained by therapeutic interventions in cases of acute pancreatitis. Another factor which explains this decrease in serum myeloperoxidase levels is the elimination of neutrophils as the main inflammatory cell in initial stages and propagation of inflammation by mononuclear cells in later phases of disease. Almost similar findings have been observed by who found high levels of inflammatory markers like pentraxin-3 (PTX-3), MPO, Procalcitonin (PCT), and C-Reactive Protein (CRP) in patient of acute pancreatitis [14].
In the present study we categorized patients of acute pancreatitis into different grades of severity by following known indicators like Ranson’s, APACHE and CT severity index scores. Whatever be the scoring system used, the patients having severe disease were found to have significantly higher levels of serum myeloperoxidase at admission when compared to those with milder form of disease. Initially high MPO levels correlated with degree of inflammation present in patients with severe disease.
The patients who developed purulent complications of pancreatitis were observed to have persistently raised MPO levels even after first week of disease onset. This is probably explained by contamination of necrotic foci occurring in patients with extensive necrosis of pancreatic tissue. Prolonged high MPO levels point to continued neutrophil activation with liberation of biologically active substances.
It was observed that patients who developed persistent organ failure had quite high serum MPO levels as compared to those who had transient or no organ failure. Repeated increases of MPO blood levels signal the development of pancreatitisassociated lung injury, or purulent-septic complications. The products formed under the influence of MPO, have protective properties; however its uncontrolled increase leads to damage of the organism’s own cells.
Serum MPO levels of patients having the worst outcome i.e. death were found to be persistently raised. The mortality from acute pancreatitis usually occurs due to severe complications whether local or systemic. There occurs intense systemic inflammatory response to pancreatic insult. In patients of acute pancreatitis who develop multi organ dysfunction syndrome, sequelae to systemic inflammatory response syndrome have worst prognosis due to intense inflammation associated tissue injury [15].
Despite a lot of research on pathophysiology, clinical presentation and treatment in patients of acute pancreatitis, there is still a lack of an ideal predictor of severity of disease. The literature is replete with researches on various predictors of severity either single- or multi-parameter, but none can be accepted as most appropriate diagnostic as well as prognostic marker.
The importance of the study is in the sense that till date, no single satisfactory marker has been identified in patients of acute pancreatitis which can diagnose this condition in patients presenting with acute abdominal pain as well as categorize these patients into different severity states. The present prospective study provides good evidence in favor of MPO to be used as a single prognostic markers in patients of acute pancreatitis at the time of presentation. The present study also supported the hypothesis of intense polymorphonuclear mediated inflammatory response in acute pancreatitis which can be downregulated by developing novel therapeutic agents with specific anti-inflammatory properties. Pentoxifylline, which has got marked anti-inflammatory properties, can be one among them.
Limitations of the study
Despite some enthusiastic results the study has some limitation as the number of cases with severe disease and complications was lower compared to other large sample size, multicenteric clinical studies; therefore, comparison of prognostic value with the current scoring systems was somewhat difficult. Moreover, raised MPO levels have been detected in various other inflammatory conditions which limit its use as a specific marker for acute pancreatitis.
Patients of acute pancreatitis who developed most severe local and/or systemic complications were found to have significantly higher serum MPO levels as compared to those with the milder form of disease. Persistently raised serum MPO levels were found in patients who either developed persistent organ failure or who succumbed to this clinically challenging disease state. However, further multicenteric clinical trials should be carried out before assigning serum MPO a great role in pancreatitis.
Research Cell, King George`s Medical University, Lucknow, UP, India for funding this work as an intramural research grant.
Late Prof Devendra Singh, Department of Surgery (General), King George`s Medical University, Lucknow, UP, India for support and guidance.
The author declare that there are no conflict of interest for the above research work
Citation: Singh VK, Sahota GS, Ali W (2021) Serial Estimation of Serum Myeloperoxidase in Patients of Acute Pancreatitis: A Pilot Study. J Oceanogr Mar Res 09(6):223.
Received: 07-Jun-2021 Accepted: 21-Jun-2021 Published: 28-Jun-2021 , DOI: 10.35248/2572-3103.21.9.223
Copyright: © 2021 Singh VK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.