Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2013) Volume 0, Issue 0
Background: The one-year survival rate of adults with the Acquired Immunodeficiency Syndrome (AIDS) after Anti Retroviral Therapy (ART) initiation and data on the efficacy of generic ART has not been well documented in India. The other objective was to see if opportunistic Infections were a significant risk factor for mortality. Methodology: Survival was studied in a prospective observational cohort study of 108 ART-naïve patients with AIDS in a South Indian state by Kaplan Meier estimates. Efficacy of different generic ART regimens was evaluated by Cox-regression analysis and multiple logistic regression analysis was performed to assess the role of opportunistic infections in mortality.
Results: The overall survival rate was 94.25% at 6 months and 88.46% at one year after ART initiation among persons who initiated therapy with CD4 counts < 200 cells/ cu.mm. There was no difference in survival between those on Efavirenz-based or nevirapine-based regimens. The mortality rate at the end of one year was 9% (nine deaths), out of which four deaths occurred within the first 90 days of ART initiation. Pulmonary Tuberculosis was a significant risk factor for mortality (OR = 4.768, p ≤ 0.05).
Conclusion: Generic ART is beneficial in a majority of AIDS patients in resource-limited settings. Pulmonary Tuberculosis is an important risk factor for mortality in AIDS patients on ART.
Keywords: AIDS; ART; Mortality; Pulmonary tuberculosis; Survival
Combination antiretroviral therapy (ART) with three or more medications for patients with the Acquired Immunodeficiency Syndrome (AIDS) has reduced morbidity and mortality [1]. The positive impact of antiretroviral therapy (ART) on HIV viral load decrease, CD4 count increase and improved survival is well documented in the developed world [1-4]. The largest populations of persons with HIV are living in resource-poor areas, where much less was known about the effect of ART on the clinical course and survival of HIV-infected persons. However, after the scaling up of ART by various international initiatives, a number of studies have come up from the developing world [5-8]. Comparatively, there are fewer published studies from India on this aspect [9]. Generic ART forms the backbone of treatment of AIDS patients in India. This is provided free of cost to the patients under the National AIDS Control Programme (NACP) [10]. The present study was therefore conducted to calculate the probability of survival among AIDS patients after one year of ART initiation, to determine the efficacy of different generic ART regimens and to see if opportunistic infections (OIs) are a significant risk factor for mortality after ART initiation.
Study design and setting
A prospective observational cohort study was conducted from August 2006 to August 2007, involving 108 ART-naïve AIDS patients attending our ART Centre at K. R. Hospital, Mysore, India. This study was approved by the ethics Committee of our institution.
After taking an informed consent for HIV testing, individuals voluntarily attending the Integrated Counseling and Testing Centre (ICTC) at the Department of Microbiology, Mysore Medical College and Research Institute, Mysore (or any of the Government designated ICTCs), underwent pre-test counselling by male or female ICTC counsellors, followed by HIV testing per strategy III of the National AIDS Control Organization (NACO) guidelines (for HIV testing) [11]. After post-test counselling, those found HIV positive were referred to the ART Centre, K. R. Hospital, Mysore Medical College and Research Institute, Mysore, where they underwent pre-ART counselling. After clinical evaluation, informed consent was taken from these patients and they were enrolled into the study if they satisfied the inclusion criteria.
Inclusion criteria
The inclusion criteria were as follows: individuals should be above 18 years of age, proven to be HIV-positive, and should not be on prior anti-retroviral therapy. Those individuals found to be HIVseronegative and those on prior ART were excluded.
The CD4/CD3 enumeration was done using the single-platform BD FACS CaliburTM machine (Becton, Dickinson and Company, San Jose, United States of America), by strictly following the manufacturer’s instructions.
Follow-up
For each patient, the clinic visit prior to initiating a new ART regimen served as the baseline, during which the CD4 counts were recorded. After initiation of ART, these patients were followed up monthly until the end of the study period.
Treatment
Those found eligible for ART as per the WHO guidelines [12], were started on anti-retroviral therapy. The anti-retroviral drugs were supplied and given free of cost by NACO under the NACP. Firstline antiretroviral-therapy regimens followed WHO guidelines. The first-line antiretroviral- therapy regimens used in this cohort were Zidovudine –based or Stavudine-based. Zidovudine based regimens consisted of Zidovudine, Lamivudine and Nevirapine/Efavirenz. Stavudine-based regimens consisted of Stavudine, Lamivudine and Efavirenz/Nevirapine.
Statistical analysis
A database entry, including patient demographics, dates of patient visits, clinical diagnoses listed at each visit and all antiretroviral medications was established for each cohort in this study attending our ART centre.
Comparison of categorical data between groups was evaluated by Fisher’s exact test. Survival after the initiation of ART was estimated using the method of Kaplan and Meier. For patients who did not reach the end point, the data were censored at the date of the last visit. Efficacy of different generic ART regimens was evaluated by Coxregression analysis.
Multiple logistic regression analysis was performed to assess the role of Opportunistic infections (OIs) in mortality. All statistical analyses were performed using SPSS software (version 16.0, SPSS, Chicago, USA). All statistical tests were two-sided and p≤ 0.05 was considered statistically significant.
Profile of the study cohort
Of the 108 ART-naïve AIDS patients, ART was initiated in a 100 patients. Eight (7.41% percent) of them met the criteria for antiretroviral therapy but were lost to follow-up before therapy could be initiated. A total of 13 patients were lost to follow-up (LFU) and a further 9 were transferred out from our ART centre to another ART centre in the surrounding districts. Figure 1 shows the profile of our study cohort.
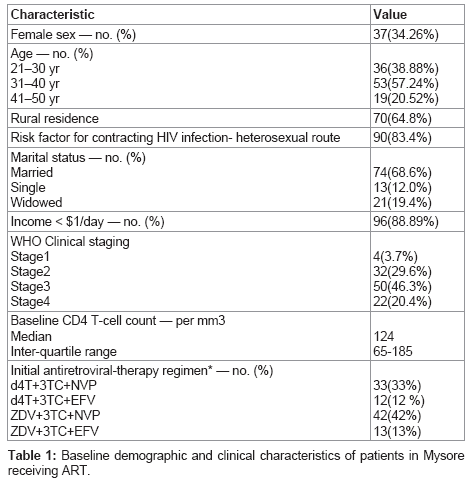
Table 1 shows the baseline demographic and clinical characteristics of patients in our study. In this cohort, 71 (65.74%) were males and 37 (34.26%) were females. 90(83.4%) acquired HIV infection via heterosexual transmission, and the median age was 35 years [inter-- quartile range- 30-39years]. The median CD4 cell count at baseline was 124 cells/cu.mm. The most common first-line regimen was zidovudine (AZT), lamivudine(3TC) and nevirapine (NVP) administered to 42 patients (42%) as a fixed-dose combination. PCP prophylaxis was given to all the patients free of cost.
Mortality
Of the hundred patients started on ART, the mortality rate at the end of one year was 9% (nine deaths), out of which four deaths occurred within the first 90 days of ART initiation. Of these nine deaths, two were due to Steven –Johnson syndrome, two were due to Tuberculosis, one each due to Cerebral toxoplasmosis, Cryptococcal meningitis and Nevirapine –rash and two were due to unknown causes.
CD4 T-Cell Response
The median increase in the CD4 T-cell count at six months was 184.5 cells per cubic millimeter (interquartile range, 165 to 258 per cubic millimeter). The CD4 T-cell count at six months was greater than the baseline value in 62 of 66 patients (93.93 percent) and remained the same or decreased from baseline in 4 (6.07 percent). The median increase in the CD4 T-cell count at 12 months was 187 cells per cubic millimeter (interquartile range, 168.75 to 217 per cubic millimeter).
Survival analysis
Figure 2 illustrates overall survival during the course of follow-up of one year. The overall survival rate was 94.25% at the end of six months of HAART and 88.46% at the end of one year of ART initiation.
Though patients on Efavirenz -based regimens tended to show better survival when compared with those on Nevirapine-based regimens, this difference was not statistically significant (hazard ratio [HR] = 1.104, 95% confidence interval [CI]: 0.671 to 1.815; P = 0.698; Figure 3).
Role of OIs in mortality
The multivariate logistic regression analysis done to assess the role of OIs in mortality showed that Pulmonary Tuberculosis was a highly significant risk factor for mortality (OR = 4.768, p = 0.05). Although statistical significance was not noted, abdominal tuberculosis (OR=6.340, p = 0.174) negatively affected survival. Table 2 shows the opportunistic infections at first admission and mortality multivariate regression analysis.
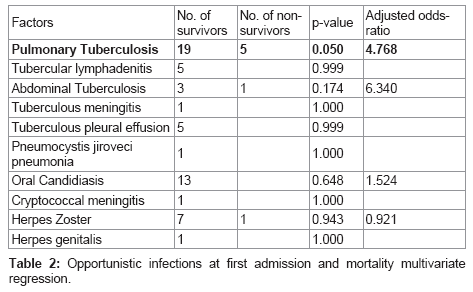
Four of the nine deaths (44.44%) which occurred after HAART initiation were due to opportunistic infections. This indicates the dominant role played by OIs in causing mortality in AIDS patients.
With an annual incidence of two million cases, India has the highest burden of tuberculosis in the world, accounting for one-third of the world’s cases [13].We have shown in our study that pulmonary tuberculosis is an important risk factor for mortality in AIDS patients.
A study from Chennai, South India showed that the most common AIDS –defining illness was Pulmonary Tuberculosis seen in 49% of the patients with a median duration of survival of 45 months [14].
In another study from Bangalore in South India, Rajagopalan et al.[15] observed that pulmonary tuberculosis (TB) at first admission (OR = 4.86) was a significant risk factor for mortality while TB in general also negatively impacted survival (p = 0.002). In a study from Uganda, Africa, the overall relative hazard for death associated with tuberculosis was 1.81 (95% CI, 1.24± 2.65). [16]. This shows TB/HIV co-infection still remains a big challenge for survival of AIDS patients.
There was a median increase of 184.5 cells per cubic millimetre in the CD4 counts after six months of HAART. Other studies from the developing world have also shown good increase in the CD4 counts after HAART initiation[5,7].
The one-year survival after HAART initiation in our study was 88.46%. Severe et al [7], reported an identical one-year survival rate of 87 percent. Good one-year survival rates have also been reported by other studies from the developing world [5,9,14]. In contrast, the mean duration of survival without antiretroviral therapy for adults in India was about 33 percent [14]. This shows the beneficial effects of HAART on survival even in resource-limited settings.
The hazard ratio for EFV-based regimens as calculated by Coxregression model was 1.104 times that for NVP-based regimens for survival. However, as the significance value was >0.1, any difference between the two regimens is due to chance. This shows that both the generic HAART regimens are equally efficacious in improving survival.
The small size of our study group and the short duration of the study period were the major limitations of our study.
More studies will be required in the area of ART in resource-limited settings, particularly in identifying easier ways of detecting resistance to anti-retrovirals, reducing the pill burden on the patients to improve compliance and to identify other optimal regimens which have better cost- effectiveness ratios.
This study has shown the short-term effectiveness of generic HAART regimens in resource-limited settings and established a strong case for further scale-up of HAART in the entire developing world.
1. To Mr. Khan Ghori for his technical support,
2. To the department of Pathology, Mysore Medical College and Research Institute, Mysore for their technical help in processing of blood samples for blood counts.
3. To the department of Medicine, Mysore Medical College and Research Institute, Mysore for their help in conducting the study.
4. To the National AIDS Control Organization for allowing us to use the facilities at The ART centre, Mysore.
5. To Dr. Noyal Maria Joseph for his help with the statistics.