Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2023)Volume 14, Issue 4
Background: Atrial Fibrillation (AF) is the most common sustained cardiac arrhythmia in clinical practice and has a well-established association with Coronary Artery Bypass Graft (CABG) surgery. Being able to predict Post- Operative Atrial Fibrillation (POAF) may improve surgical outcomes. This study aims to understand the efficacy of incorporating intraoperative medication data to predict first-time POAF in patients undergoing CABG surgery.
Methods: This study aims to understand the efficacy of incorporating intraoperative medication data to predict first-time POAF in patients undergoing CABG surgery. A large cohort of 3807 first-time CABG patients with no known history of atrial fibrillation was retrospectively assembled to study factors that contribute to occurrence of post-operative atrial fibrillation, in addition to testing models that may predict its incidence. To do so, several clinical features with established relevance to POAF were extracted from the electronic health record, along with a record of medications administered intra-operatively. Tests of performance with logistic regression, decision tree, and neural network predictive models showed slight improvements when incorporating medication information.
Results: Analysis of the collected set of clinical and medications data indicate that there may be effects contributing to POAF incidence captured in the medication administration records. However, a definitive causal relationship between the medications and POAF incidence is not established.
Conclusions: Our results show that improved predictive performance is achievable by incorporating a record of medications administered intra-operatively, but further investigation is needed to understand the implications of this for clinical practice.
Atrial fibrillation; Coronary artery bypass graft; Enterprise data warehouse; Chronic obstructive pulmonary disease; Least absolute shrinkage and selection operator
Atrial Fibrillation (AF) is the most common sustained cardiac arrhythmia in clinical practice and has a well-established association with Coronary Artery Bypass Graft (CABG) surgery. Several studies have shown greater morbidity, mortality, and increased utilization of care resources in patients with Post-Operative Atrial Fibrillation (POAF) [1-5]. Previous efforts to predict POAF have explored well-known clinical risk factors, including age, race, BMI, blood pressure, tobacco use, and history of myocardial infarctions and AF [6-8]. Other factors such as genetic risk associations with AF, various biomarkers, Electrocardiogram (ECG) data, and cardiovascular disease medication usage have been incorporated with varying degrees of success in improving model accuracy [5-13]. Studies incorporating many or all these factors have presented models having moderate predictive ability, with areas under the curve Appropriate Use Criteria (AUCs) in the range of 0.6- 0.8 [5,6,10,14]. Prediction of new-onset AF in AF-naïve patients, however, has generally been more challenging, with correspondingly lower (<0.7) AUC performance [10,14]. The physiologic stress of surgical operations can result in AF-naïve patients, generally healthier than patients with past AF, nevertheless developing AF post-surgery [15]. However, prior AF has consistently been a strong predictor of POAF and predicting POAF in AF-naïve cohorts remains challenging.
Better understanding POAF in AF-naïve patients would offer potential paths for improving outcomes among patients that are perceived with lower risk. Prior studies have highlighted the possible contributions of hemodynamic instability and inflammation in development of POAF [15-17]. However, there has been relatively limited study of how perioperatively administered medications affect AF incidence. The incorporation of medications information into prior work has mostly been limited to studying beta-blockers. Here, we have leveraged Electronic Medical Records (EMRs), which capture a rich set of clinical features that can be combined with previously identified predictors in order to improve prediction accuracy. We hypothesize that the inclusion of other medications typically used around the time of CABG surgery may improve the ability to predict POAF. As an initial exploration of how intraoperative medications information affects probability of POAF occurrence, we assembled a retrospective data set of first-time CABG patients with no history of AF. We included traditional clinical risk factors, such as, smoking history and patient co-morbidities. Additionally, we included information about medication administrations on the day of surgery for CABG procedures. We tested the collected features as variables in multiple prediction models to observe the potential for intraoperative medications to provide a more accurate model for new-onset POAF in CABG patients.
Study population
The study cohort was assembled retrospectively from de-identified patient EMR using the Enterprise Data Warehouse (EDW) of North-western Memorial Hospital (NMH) in Chicago, Illinois, USA. Patients undergoing first-time CABG surgery between January 2003 and July 2019 were identified by ICD-9, ICD-10 and CPT billing codes. First time procedures were identified by absence of previous records for the surgery in the EDW, and only such patients were included to create an AF naïve cohort. Cases of post-operative AF were confirmed by any diagnosis via billing codes or ECG reading notes prior to their discharge. Additionally, patients who did not have any medication information recorded in the EDW were removed. Analysis for this study was conducted on de-identified records with Institutional Review Board (IRB) exemption for informed consent.
Clinical factors data
Patient demographics and other potential clinical predictors of POAF reported in previous literature were extracted from the EDW. The variables include age, gender, race, and smoking history, diagnosis for Chronic Obstructive Pulmonary Disease (COPD), prior AF, hypertension, Diabetes Mellitus (DM), and Pulmonic Regurgitation (PR) interval. Pre and post-operative ACE inhibitors, aspirin and beta-blocker usage were also included as binary variables. PR intervals were extracted from the NMH cardiology department’s ECG data management system. Post-operative beta-blocker use was defined as any use of beta-blocker between surgery and discharge date. Diagnoses for co-morbidities such as COPD were extracted using ICD-9/10 codes. Procedure codes (CPT, ICD9PCS, ICD10PCS) were used to determine concurrent valve repair or replacement surgery on the day of CABG by matching additional procedure codes on the same date. Valve surgeries were categorized based on the valve (mitral versus aortic) operated on. Patients with procedure codes for both valves on the day of CABG were given a third category (both valves). Dummy variables were created for the presence of each co-morbidity. Race was treated as a four-class categorical variable: Caucasian, African American, Asian and others.
Intra-operative medication data
Patient medication records were extracted from the EDW. Intraoperative medication was determined as medication administered on the day of surgery. Medication quantities were recorded in the database as either one-time dose (e.g., “5 mg IV push”) or continuous, finite-duration administration (e.g., “250 ml/hour, 60 minutes”). All records included start/end times. Similar medications were aggregated based on their RXCUI Ingredient code. For instance, different sodium bicarbonate salt solutions were aggregated into the same class of medications. The top 30 most common medications were selected based on their incidence rate in the cohort population. Dosages were imputed for medication records lacking dosage information (2% of dataset) using most common dosage for each type.
Data preparation
Four data representations were created for model training and testing. The first representation contained only the traditional clinical variables. The second included the clinical variables and binary variables indicating whether each of the 30 most-common medication categories was administered. The third representation included the clinical variables and continuous-valued representations of the medication administrations. The final representation included a matrix composed of the temporal sequence of the intraoperative medication. For each patient and each medication, dosages over 24-minute segments on the day of surgery were specified. Missing PR interval values were imputed using Predictive Mean Matching (PMM) over 10 iterations. In our third dataset, medication dosages were scaled relative to the median, since median dosages of different medications vary greatly. All datasets were split randomly at a 7:3 ratio into training and testing datasets.
Predictive modelling
Four classification methods were selected to explore the utility of the addition of medication for predicting POAF, which include Logistic Regression (LR), Least Absolute Shrinkage and Selection Operator (LASSO), Extreme Gradient Boosting (XGBoost) and a Neural Network (NN) architecture (Figure 1). Given the large number of predictors in our model, LASSO was performed to improve model performance and interpretability. The LASSO model was optimized via selecting the penalty parameter yielding the greatest AUC via 10-fold cross validation. XGBoost is a gradient-boosted decision tree method [18]. Hyper parameters were tuned by grid search for highest test AUC values. The LR, LASSO, and XGBoost models were each applied to the first three data representations: clinical variables only, clinical plus binary medications, and clinical plus medication dosages. We evaluated the utility of including medication information for POAF prediction by comparing the AUC for each method and each dataset [19]. The model trained on the clinical variables only dataset served as the benchmark for each method. The NN model was applied to the clinical plus temporal medication dosage data. To use both the structure of sequential medication dosage measurements and clinical variables, we created a mixed input neural network architecture combining a perceptron with several fully-connected layers [20,21] (Figure 1). The NN model was used to extract temporal patterns for each medication with a temporal convolution layer to produce a tensor of outputs, followed by max pooling and relu activation [22]. The convolutional branch output was flattened and combined with the perceptron output from patient clinical information. The combined dense layer then was followed by two additional dense layers, and final output generated by sigmoid activation function. Binary cross entropy was used as the loss function. Batch size, learning rate, optimizer, neuron/layer/filter count, max pool size, number of epochs were tuned to optimize the performance of the model. Drop out and batch normalization were also tuned to avoid overfitting. Statistical software R (Version 4.0.2) and Python 3.7 was used for the data processes and statistical analysis. LASSO was performed using the R glmnet package [18]. XGBoost was performed and tuned using the python xgboost and sklearn package respectively [19]. The NN model was performed and tuned using Keras in Python 3.7 [20-22]. All performance was assessed by prediction AUC on a test subset of data, with a split of 70% used for training and 30% used for testing.
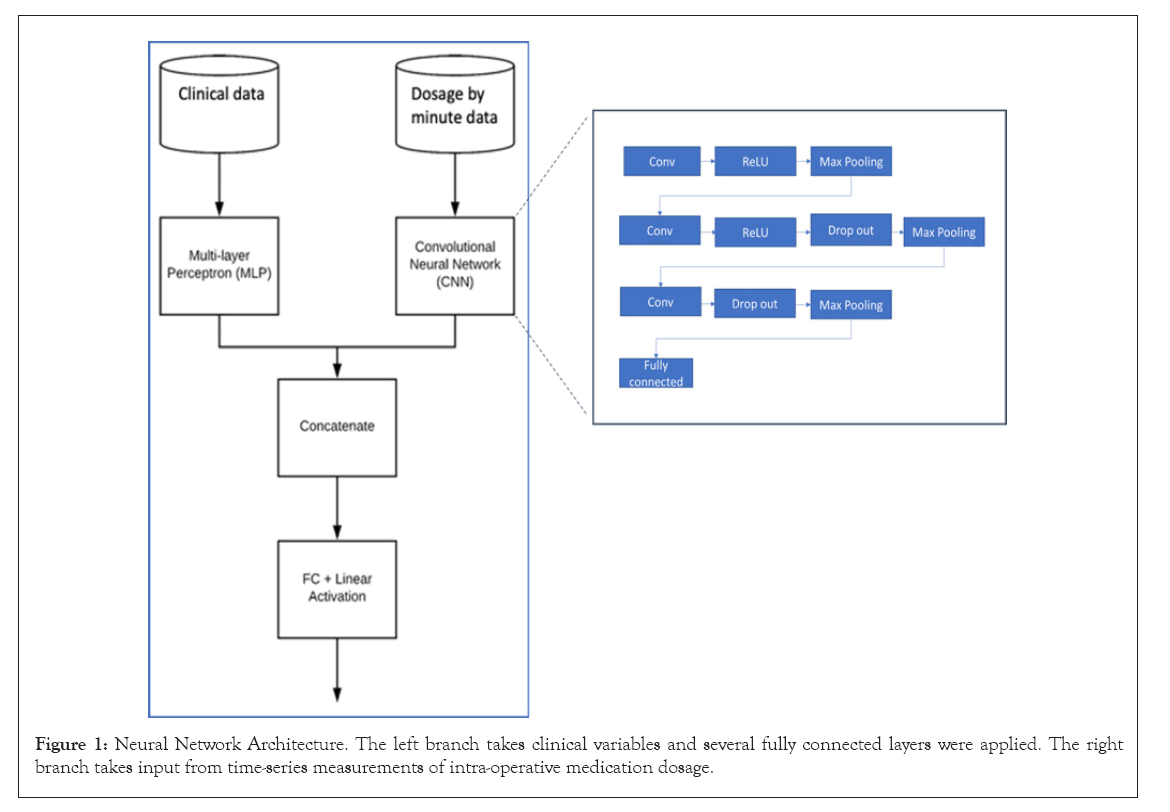
Figure 1: Neural Network Architecture. The left branch takes clinical variables and several fully connected layers were applied. The right branch takes input from time-series measurements of intra-operative medication dosage.
Patient characteristics and medications
Baseline patient characteristics are presented in Table 1. Our AF naïve study population included 3807 unique patients, POAF was observed in 789 (21%) patients (cases; all others were controls). In our study population, the mean age was 66 years, 75% of patients were male, 55% were hypertensive, and 26% were diabetic (Table 1). Mitral valve disorders occurred in 10% of case patients, significantly different from control patients (5%, p<0.01). Average PR intervals were higher in cases at 179 ms, whereas in controls, average PR intervals were 170 ms. The 30 most common intraoperative medications based on the incidence rate among the entire patient population are shown in Supplementary Table 1. Many intraoperative medications were found to have statistically significant differences in administration patterns between case and control patients. Most of those medications also had statistically significant different administered dosages between groups, except for epinephrine.
| Variables | Overall (N=3807) | Cases (N=789) | Controls (N=3018) | P-Value |
|---|---|---|---|---|
| Average Age y, Mean (SD) | 66.3 (10.7) | 70.6 (9.58) | 65.1 (10.6) | <0.01 |
| Gender Female | 2589 (75.1%) | 585 (74.1%) | 2274 (75.3%) | 0.516 |
| Smoking Status | 630 (16.5%) | 116 (14.7%) | 514 (17.0%) | 0.13 |
| Chronic Obstructive Pulmonary Disease | 453 (11.9%) | 103 (13.1%) | 350 (11.6%) | 0.287 |
| Hypertension | 2084 (54.7%) | 455 (57.7%) | 1629 (54.0%) | 0.07 |
| Diabetes | 997 (26.2%) | 186 (23.6%) | 811 (26.9%) | 0.067 |
| Mitral Valve Disease | 221 (5.8%) | 82 (10.4%) | 139 (4.6%) | <0.01 |
| Heart Failure | 747 (19.6%) | 177 (22.4%) | 570 (18.9%) | 0.029 |
| Myocardial Infarction | 2510 (65.9%) | 506 (64.1%) | 2004 (66.4%) | 0.248 |
| Pre-Operative ACEi Use | 1283 (33.7%) | 311 (39.4%) | 972 (32.2%) | <0.01 |
| Pre-Operative Aspirin Use | 1586 (41.7%) | 330 (41.8%) | 1256 (41.6%) | 0.948 |
| Pre-Operative Beta-Blocker Use | 1461 (38.4%) | 345 (43.7%) | 1116 (37.0%) | <0.01 |
| Post-Operative ACEi Use | 1921 (50.5%) | 392 (49.7%) | 1529 (50.7%) | 0.653 |
| Post-Operative Aspirin Use | 2421 (63.6%) | 471 (59.7%) | 1950 (64.6%) | 0.012 |
| Post-Operative Beta-Blocker Use | 3011 (79.1%) | 586 (74.3%) | 2425 (80.4%) | <0.01 |
| Average PR Interval, Mean (SD) | 172 (31) | 179 (38) | 170 (29) | <0.01 |
| Race | - | - | - | 0.014 |
| White | 2882 (75.7%) | 630 (79.8%) | 2252 (74.6%) | - |
| Black | 287 (7.5%) | 45 (5.7%) | 242 (8.0%) | - |
| Other | 180 (4.7%) | 28 (3.5%) | 152 (5.0%) | - |
| Unknown | 458 (12.0%) | 86 (10.9%) | 372 (12.3%) | - |
Table 1: AUC values of the predictive models in the testing dataset.
Prediction modelling
AUC values of prediction models are shown in Table 2. Overall, the inclusion of intraoperative medications provided some improvement in the predictive power of models compared to the models that only included traditional clinical variables. In models that used LR and LASSO, clinical only models had AUC values around 0.67-0.69 (95% CI: 0.63-0.73), while models that included any form of medication information had AUC values of 0.70-0.71 (95% CI: 0.66-0.75). The XGBoost models showed similar improvements; the clinical variables only model had AUC of 0.65 (95% CI: 0.63-0.67), and models with medication information had a higher AUC of 0.67-0.68 (95% CI: 0.64-0.70), although they had an overall lower predictive power than LR. However, there was no clear performance differences between the models that used binary medication variables and the models that used medication dosages in our LR, LASSO, and XGBoost models. The NN model, using temporal medication sequence data, yielded AUC of 0.70, similar performance to that of the LR and LASSO models that used only aggregate dosage data for intraoperative medications. LR, LASSO, and NN had overall better performance than XGBoost.
| Prediction Method | AUC by Dataset Type (95% Confidence Interval) | |||
|---|---|---|---|---|
| Clinical Only | Binary Meds | Med Dosages | Med Sequence | |
| LR | 0.67 (0.63-0.71) | 0.70 (0.66-0.73) | 0.70 (0.66-0.73) | N/A |
| LASSO | 0.69 (0.65-0.73) | 0.71 (0.67-0.75) | 0.71 (0.67-0.75) | N/A |
| XGBoost | 0.65 (0.62-0.67) | 0.67 (0.64-0.69) | 0.68 (0.65-0.70) | N/A |
| Neural Network | N/A | N/A | N/A | 0.70 (0.69-0.71)* |
Table 2: AUC values of the predictive models in the testing dataset.
Coefficients of LR and LASSO models are presented in Table 3. Age and male sex were found positively associated with the development of POAF. Longer PR intervals was positively associated with our outcome. Having mitral valve disease was also a strong positive predictor for development of POAF, while significant relationships were not found in other comorbidities. Pre-operative beta-blocker use was found to be positively associated with POAF incidence. Having concurrent valve repair or replacement surgeries, particularly on the aortic valve or on both valves, was found to have a positive relationship with POAF onset. Medications such as albumin, cefazolin, and chlorhexidine gluconate were found to be negatively associated with development of POAF across all or most LR and LASSO models. XGBoost feature-importance plots are presented in (Figure 2a). Features such as age and average PR interval are consistently ranked highest across all models. Features highlighted in the XGBoost medication dosage model exhibit significantly different variables compared to the other two models. Medications such as albumin, aspirin, dextrose, fentanyl, and propofol were considered as most important features in addition to age and PR interval. The XGBoost importance rankings for each predictor in each model are shown in (Figure 2b).
| Variables | Clinical Model (A) | Binary Medication Model (B) | Medication Dosage Model (C) | LASSO (D) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | CI | P-Value | OR | CI | P-Value | OR | CI | P-Value | (A) OR | (B) OR | (C) OR | |
| Age | 1.04 | 1.04-1.05 | < 2e-16 | 1.05 | 1.04-1.06 | <0.01 | 1.05 | 1.04-1.06 | <0.01 | 1.04 | 1.04 | 1.04 |
| Gender Male | 1.19 | 0.98-1.45 | 0.08 | 1.24 | 1.02-1.52 | 0.03 | 1.22 | 1.00-1.50 | 0.05 | - | - | - |
| Smoker | 1.03 | 0.81-1.30 | 0.83 | 0.98 | 0.76-1.25 | 0.87 | 0.98 | 0.77-1.25 | 0.9 | - | - | - |
| COPD | 1.13 | 0.87-1.45 | 0.36 | 1.14 | 0.88-1.48 | 0.31 | 1.15 | 0.89-1.49 | 0.28 | - | - | - |
| Hypertension | 0.88 | 0.72-1.08 | 0.23 | 1.02 | 0.84-1.23 | 0.88 | 0.99 | 0.82-1.20 | 0.96 | - | - | - |
| Diabetes | 1.02 | 0.85-1.23 | 0.81 | 0.85 | 0.69-1.05 | 0.14 | 0.86 | 0.70-1.06 | 0.15 | - | - | - |
| Mitral Valve Diseases | 1.63 | 1.16-2.27 | 0 | 1.65 | 1.16-2.34 | 0.01 | 1.66 | 1.17-2.34 | <0.01 | - | - | - |
| Heart Failure | 1.06 | 0.86-1.31 | 0.57 | 0.95 | 0.76-1.19 | 0.67 | 0.96 | 0.77-1.19 | 0.69 | - | - | - |
| Myocardial Infarction | 0.86 | 0.72-1.04 | 0.12 | 0.91 | 0.75-1.11 | 0.35 | 0.91 | 0.75-1.10 | 0.33 | - | - | - |
| Post-Operative ACEi Use | 1.09 | 0.90-1.32 | 0.37 | 0.95 | 0.78-1.16 | 0.59 | 0.96 | 0.79-1.18 | 0.71 | - | - | - |
| Post-Operative Aspirin Use | 1 | 0.82-1.23 | 1 | 0.98 | 0.79-1.22 | 0.87 | 0.99 | 0.80-1.23 | 0.93 | - | - | - |
| Post-Operative Beta-Blocker Use | 0.84 | 0.66-1.07 | 0.16 | 1.02 | 0.79-1.31 | 0.9 | 1 | 0.78-1.29 | 1 | - | - | - |
| Pre-Operative ACEi Use | 1.18 | 0.96-1.46 | 0.12 | 1.07 | 0.86-1.33 | 0.54 | 1.09 | 0.88-1.35 | 0.43 | - | - | - |
| Pre-Operative Aspirin Use | 0.83 | 0.68-1.01 | 0.06 | 0.85 | 0.69-1.04 | 0.12 | 0.83 | 0.67-1.02 | 0.08 | - | - | - |
| Pre-Operative Beta-Blocker Use | 1.33 | 1.08-1.64 | 0.01 | 1.34 | 1.07-1.66 | 0.01 | 1.34 | 1.08-1.66 | 0.01 | - | - | - |
| Concurrent Mitral Valve Operation | 1.66 | 1.18-2.32 | 0 | 1.34 | 0.94-1.90 | 0.1 | 1.34 | 0.36-1.01 | 0.1 | 1.38 | 1.06 | - |
| Concurrent Aortic Valve Operation | 1.74 | 1.41-2.13 | 0 | 1.57 | 1.26-1.94 | <0.01 | 1.57 | 0.30-0.90 | <0.01 | 1.71 | 1.44 | - |
| Concurrent Both Valve Operation | 3.32 | 2.04-5.39 | 0 | 2.7 | 1.63-4.46 | <0.01 | 2.59 | 0.23-0.64 | <0.01 | 2.69 | 1.97 | - |
| Average PR Interval (per 5 ms) | 1.02 | 1.00-1.03 | 0.01 | 1.02 | 1.01-1.03 | <0.01 | 1.02 | 1.01-1.03 | <0.01 | 1.01 | 1.01 | - |
| Albumin (g) | - | - | - | 0.63 | 0.51-0.78 | <0.01 | 0.96 | 0.95-0.98 | <0.01 | - | 0.77 | - |
| Aminocaproic Acid (g) | - | - | - | 0.93 | 0.65-1.34 | 0.71 | 1 | 0.97-1.03 | 0.94 | - | - | - |
| Aspirin (mg) | - | - | - | 1.12 | 0.92-1.36 | 0.26 | 1.01 | 0.99-1.02 | 0.46 | - | - | - |
| Calcium Chloride (mg) | - | - | - | 1 | 0.80-1.26 | 0.98 | 1 | 0.99-1.02 | 0.54 | - | - | - |
| Cefazolin (mg) | - | - | - | 0.71 | 0.52-0.96 | 0.03 | 0.98 | 0.96-1.00 | 0.02 | - | 0.83 | - |
| Cefuroxime (g) | - | - | - | 1.07 | 0.86-1.34 | 0.53 | 1.01 | 0.98-1.03 | 0.63 | - | 1.15 | - |
| Chlorhexidine Gluconate (mg) | - | - | - | 0.52 | 0.34-0.78 | <0.01 | 0.94 | 0.90-0.97 | <0.01 | - | 0.63 | - |
| Cholecalciferol (g) | - | - | - | 0.99 | 0.78-1.27 | 0.95 | 1 | 0.97-1.02 | 0.79 | - | - | - |
| Dextrose (g) | - | - | - | 1.08 | 0.76-1.52 | 0.67 | 1.01 | 1.00-1.03 | 0.17 | - | 1.19 | - |
| Epinephrine (mg) | - | - | - | 1.16 | 0.82-1.66 | 0.4 | 1 | 0.99-1.01 | 0.74 | - | - | - |
| Fentanyl (mg) | - | - | - | 1.16 | 0.90-1.49 | 0.26 | 1 | 0.99-1.00 | 0.24 | - | - | - |
| Furosemide (mg) | - | - | - | 1.17 | 0.89-1.54 | 0.26 | 1.01 | 0.99-1.03 | 0.25 | - | - | - |
| Heparin (g) | - | - | - | 1.08 | 0.65-1.75 | 0.75 | 1.04 | 1.00-1.09 | 0.06 | - | - | - |
| Hetastarch (g) | - | - | - | 0.75 | 0.55-1.00 | 0.06 | 0.97 | 0.94-1.00 | 0.08 | - | - | - |
| Insulin (mg) | - | - | - | 1.14 | 0.81-1.61 | 0.46 | 1.01 | 0.98-1.04 | 0.39 | - | - | - |
| Lidocaine (mg) | - | - | - | 1.17 | 0.91-1.49 | 0.22 | 1.01 | 0.99-1.04 | 0.36 | - | 1.03 | - |
| Magnesium Sulfate (g) | - | - | - | 1.08 | 0.84-1.38 | 0.56 | 1 | 0.99-1.01 | 0.66 | - | - | - |
| Midazolam (mg) | - | - | - | 1.11 | 0.82-1.51 | 0.51 | 1 | 0.99-1.00 | 0.39 | - | - | - |
| Mupriocin (mg) | - | - | - | 1.06 | 0.84-1.34 | 0.61 | 1 | 0.98-1.01 | 0.82 | - | - | - |
| Nitroglycerin (mg) | - | - | - | 1.05 | 0.78-1.41 | 0.74 | 1 | 0.99-1.01 | 0.78 | - | - | - |
| Norco (mg) | - | - | - | 1 | 0.66-1.54 | 0.98 | 0.99 | 0.95-1.04 | 0.8 | - | - | - |
| Norepinephrine (mg) | - | - | - | 1.29 | 0.85-1.98 | 0.24 | 1 | 0.99-1.01 | 0.8 | - | - | - |
| Ondansetron (mg) | - | - | - | 1.19 | 0.81-1.74 | 0.36 | 1.02 | 0.98-1.06 | 0.37 | - | - | - |
| Potassium Chloride (mg) | - | - | - | 0.92 | 0.73-1.15 | 0.45 | 1 | 0.99-1.01 | 0.99 | - | - | - |
| Propofol (mg) | - | - | - | 1.32 | 0.98-1.78 | 0.07 | 1.02 | 1.00-1.03 | 0.01 | - | - | - |
| Protamine Sulfate (mg) | - | - | - | 0.61 | 0.39-0.94 | 0.03 | 0.98 | 0.94-1.03 | 0.48 | - | - | - |
| Rocuronium (mg) | - | - | - | 0.77 | 0.35-1.69 | 0.51 | 0.98 | 0.92-1.04 | 0.44 | - | - | - |
| Sodium Bicarbonate (mg) | - | - | - | 1.25 | 0.99-1.57 | 0.06 | 1.02 | 1.00-1.04 | 0.1 | - | 1.21 | 1.02 |
| Tylenol (mg) | - | - | - | 1.12 | 0.81-1.56 | 0.48 | 1.02 | 0.99-1.05 | 0.15 | - | - | 1 |
| Vancomycin (g) | - | - | - | 0.92 | 0.73-1.16 | 0.47 | 1 | 0.99-1.01 | 0.64 | - | - | - |
Table 3: Odds Ratios of the a) Clinical Only Model, b) Binary Medication Model, c) Medication Dosage Model, and d) the Model with variable selection using LASSO. Odds Ratios that are statistically significant are highlighted in black.
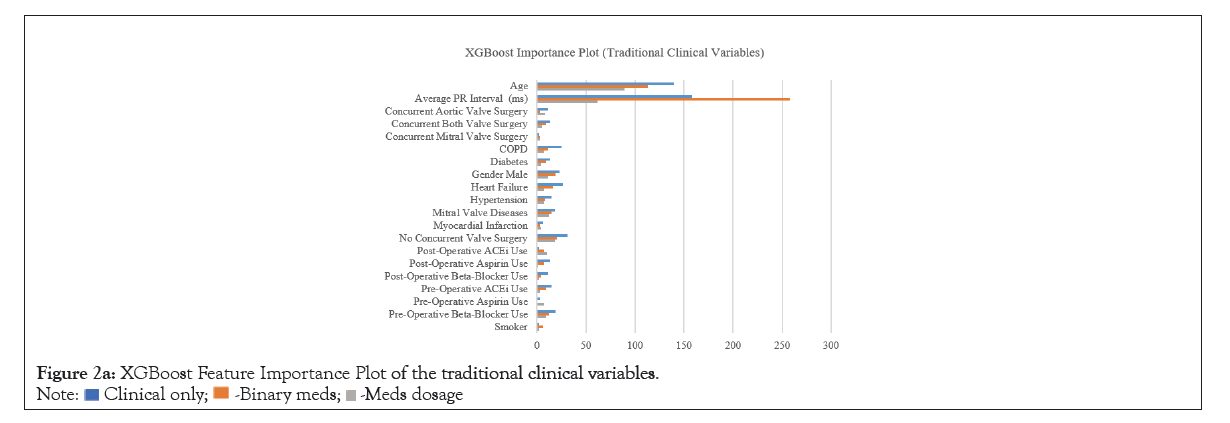
Figure 2a: XGBoost Feature Importance Plot of the traditional clinical variables.
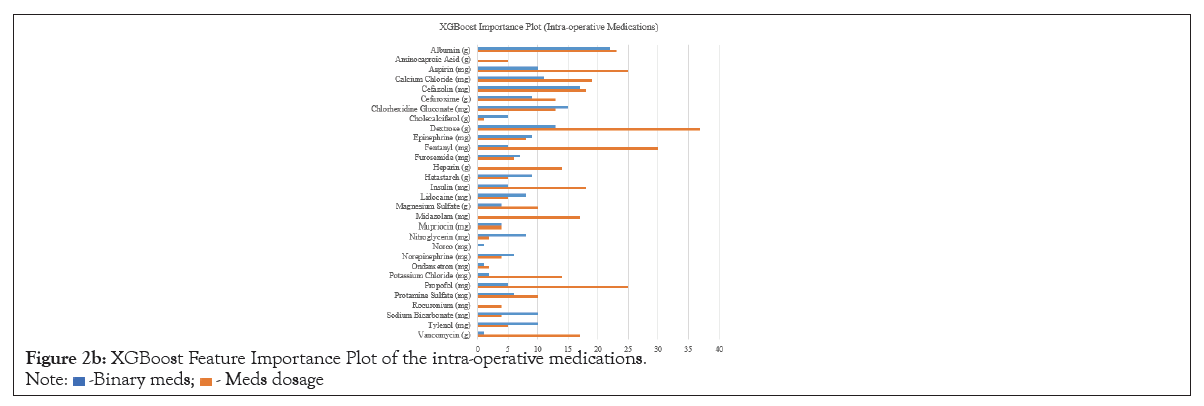
Figure 2b: XGBoost Feature Importance Plot of the intra-operative medications.
We have presented an exploration of incorporating intraoperative medications to improve the accuracy of models that predict POAF in patients receiving CABG surgery, testing the hypothesis that intraoperative medication information may be used to improve prediction of new-onset POAF incidence. Leveraging the rich clinical information available in the EMR, we constructed a large study cohort dataset, including 3,807 first-time AF CABG patients. The dataset included patient comorbidities and intraoperative medication administration profiles. Four statistical and machine learning methods were trained and tested on different representations of our dataset with varying levels of information. LR and LASSO models trained with basic clinical information had AUC values in the ranges similar to those reported in other studies with different feature sets, while XGBoost performed comparatively worse. Incorporation of intraoperative medications improved predictive accuracy (AUC) across all models. The relative performances indicate that intra-operative medications data do influence predictive performance, but the time-series medication data did not provide more predictive information than aggregated dosages did.
In our LR and LASSO models, age was the most significant predictor of POAF, which is consistent with findings of other studies [6,12]. Mitral valve disease was found be a significant predictor of POAF in our patient cohort. Other cardiovascular conditions, such as myocardial infarctions, heart failure, hypertension and several other comorbidities, including, diabetes and COPD contributed to the predictive ability of the model but were not found to be statistically significant, as has also been observed in other studies. Our model showed longer PR intervals are a positive predictor for POAF. This is also consistent with the results of previous studies [23-25]. In our models, some variables had coefficients that suggested inverted effects towards POAF based on previous reports. For instance, smoking status and hypertension predictors consistently showed negative relationships towards POAF across our two models. The incidence rate of hypertension (58% vs. 54%) and current smoker (15% vs. 17%) were higher in the control group compared to the case group, though both were not statistically significant differences. The study cohort was mostly matched in incidence for clinical POAF risk factors found in other studies, such as smoking, hypertension and diabetes, but effects observed were not significant for many of these variables [6,12]. The cohort in this study was AF-naïve, which differs from some previous studies. Medication information has been investigated to a limited extent previously in relations to POAF. Pre-operative and post-operative beta-blocker use had been found to have an effect on the development of POAF. In our models, we did find a negative relationship between post-operative beta-blocker use and onset of POAF, but no significant relationship was observed with pre-operative use. This corroborates knowledge of beta-blockers’ prophylactic properties-with regard to AF [15,16,26-29]. Note that although we attempted here to examine factors contributing to POAF incidence, our dataset did not permit clear distinction of post-operative beta-blocker use before or after the onset of POAF, which tends to confound the observed effects. It is possible that some post-operative beta-blocker use was in response to AF incidence, which would have increased observed rates of use in our case group and diminished observed differences between cases and controls.
The baseline predictive accuracy from models using only clinical data variables is comparable to that shown in other studies of AF-naive CABG patient populations, which have demonstrated predictive ability with AUC in the range of 0.60-0.68 [10-13]. The improvement in predictive accuracy after incorporating intraoperative medications suggests a potential effect of common intraoperative medications on development of POAF after CABG surgery. However, lack of difference in predictive performance between models using binary indicator variables for intraoperative medications and models using net dosages, with LR, or time-sequence information, in a neural network, suggests that the binary characterization of intraoperative medications largely describes whatever effect they have. It may be that medicines’ sodium or mineral content influences patient hydration or isotonicity to influence heart muscle function [30]. Alternatively, it may be that medications themselves do not directly affect POAF incidence; instead, they may be indicating clinical circumstances corresponding to more physiologically stressful operative conditions. For example, prior studies have shown clamp time during CABG predictive of POAF [31,32]. Here we did not have access to operation duration information, but medication administration profiles may be dependent upon it or other factors. There was positive association of POAF with undergoing of concurrent valve operations (e.g., mitral valve replacement), which would cause more physiologic stress. Significant associations with POAF of medication administrations that were observed concomitantly with concurrent valve operations may represent independent effects, or they may be capturing secondary procedural influences. For example, from an informatics perspective, medications administrations could be capturing specific pre-operative procedures of patients or variations in procedure by different care teams. Overall, the exact nature of causality between these medications and development of POAF is unclear. Furthermore, there may be inaccuracies in some of the recorded quantities administered, as they are derived from clinicians’ manual input of start and stop times in the electronic record, rather than a direct measurement of the quantity. While there could be pharmacological or other influences on patients’ physiology captured by medications information, a study with more directly-recorded data for medications would be more effective in testing this hypothesis. The results presented in this study indicate that deeper understanding of the causes for POAF and potential for improving clinical prediction models would be accessible through further investigation of medication administrations and other intra-operative procedures for AF-naive CABG patients.
This study used a retrospective data set for first-time CABG patients to investigate factors associated with POAF and potential predictive performance of several prediction models. Across models, advanced age, mitral valve disease, and increased average ECG PR interval were significantly associated with increased POAF incidence, which is consistent with the findings of other studies. Additionally, incorporation of intra-operative medication administration data was shown to improve predictive performance of all models tested. The findings presented here contribute to general understanding of POAF as having complex, multifarious influences. They indicate that improving performance for models to predict POAF incidence is possible by introducing information about medications administered intra-operatively, and they demonstrate the potential effects upon POAF incidence of several commonly used medications. However, this study did not identify causal relationships between the medications administered and POAF outcomes. Generally, as POAF is associated with higher care resource usage, the ability to predict and mitigate its incidence would be valuable. The results of this investigation can support further study into the role of intra-operative medications, potentially enabling identification of operational changes that may decrease POAF incidence.
No competing interests are reported. Research did not involve human subject’s research and thus no consent or ethics approval needed.
No funding was involved for this research.
J.Y., E.J., Y.D. all contributed to the data extraction, data cleaning, data analysis, writing and editing the manuscript. S.Z., D.M., M.E., A.K. provided technical or clinical guidance on the project, and contributed to editing of the manuscript
The data is not public because it is private health data provided by North-western Medicine.
Citation: Yu J, Johnson E, Deng Y, Zhang S, Melnick DS, Etemadi M, et al (2023) Significance of Intraoperative Medication Data and Model Selection for Predicting Postoperative First-Time Atrial Fibrillation. J Clin Exp Cardiolog.14:790.
Received: 11-May-2023, Manuscript No. JCEC-23-24046 ; Editor assigned: 13-May-2023, Pre QC No. JCEC-23-24046 ; Reviewed: 31-May-2023, QC No. JCEC-23-24046 ; Revised: 08-Jun-2023, Manuscript No. JCEC-23-24046 ; Published: 16-Jun-2023 , DOI: 10.35248/2155-9880.23.14.790
Copyright: © 2023 Yu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.